November 18, 2022
Natural Immunity Provides Far Better Protection than Was Previously Thought and Creates Vaccine Hesitancy
By specialist in general medicine (GP) Claus Hancke, MD, FACAM, physiotherapist Michael Schultz, PT, and statistician Max Schmeling.
Are you afraid of viruses? Of another shot? Need a booster? Are you in doubt?
Decades of studies now show that there may be no reason to be so afraid of viruses.
In 2008, researchers made an astonishing observation in human blood 196. It was investigated whether 32 elderly people, born in or before 1915, had been infected with the 1918 Flu, the “Spanish Flu”.
What was found in the blood? Well, all of them had remnants of the virus from 1918, and 7 even had memory cells that could still activate antibodies against the virus. This is a sign of lifelong immunity.
These findings conflict with the narrative we have been given that natural immunity after infection is short-lived.
During the first wave of Covid-19, the concept of natural infection immunity was downplayed, and artificial vaccine immunity was in focus 134.
In terms of health policy, vaccine immunity was even attributed a longer duration than the natural infection immunity, even though it’s actually the other way around.
Those in power wanted to create fear in the population in order to motivate us to comply with restrictions and vaccination.
Officially, the repetitive message was that “we are only protected for 5 months after infection” 220 and “unvaccinated people live a dangerous life” 1.
However, this goes against solid scientific data! Therefore, we have found it necessary to document the effect of infection immunity based on the largest collection of literature on the subject known to us, over 200 recognized studies.
A Danish state funded fact-checking media1 has also, unsuccessfully, tried to get the Danish Health Authorities to document their claim about “the dangerous unvaccinated life”.
It has always been possible to find virus residues in humans, because we are constantly exposed to viruses without getting sick. This is nothing new. What’s new is that we have started to “name” variants and now suddenly have to fear something that has always happened.
But is this really justified?
In this article, we will demystify the horror stories we were told, hoping that we will never again experience such a disproportionate, unreasonable, expensive, and socially harmful 55, 218 panic reaction that we’ve had to endure.
The Studies
3 decades of studies show that natural infection immunity is long-lasting 2, 19, 25, 47, 54, 64, 72, 74, 102, 103, 109, 113, 143, 151, 155, 170, 178, 179, 190, 248, often lifelong 151, 196, stronger 2, 4, 16, 19, 23, 29, 41, 53, 62, 63, 64, 75, 81, 97, 109, 112, 127, 135, 154, 155, 185, 218, 233, 235, 240, 243, and broader 30, 53, 59, 59, 84, 94, 121, 154, 156, 162, 168, 171, 192, 218, 219 than artificial vaccine immunity 245, 249. This means that we are also protected against severe symptoms, in case of infection with variants or related viruses 197, 246. Some studies show at least 15 – 22 months of immunity 3, 15, 50, 19, 112, 241, 247 from the first Covid cases. Others show immunity 17 years after infection with SARS-CoV-1 6, 53, 139, 170, 178.
A study in 12 million subjects showed a “small risk” of new infection after the first infection11 due to natural immunity. Studies falsely consider positive PCR tests in healthy people as “disease cases” 42. This is nonsense when they are not sick. Eighty percent of those infected with SARS-CoV-2 do not develop disease 213.
Immunity is the body’s response to infection and ensures none or mild symptoms the next time we encounter the same or a similar virus 231, 232. When you are immune, you can be infected, but you do not get ill 9, 78, 118, and you rarely infect others 34, 122, 137, 141. A positive test does not mean that you are sick or can infect other people, and vaccines do not prevent infection either, despite what the authorities claim.
Studies show that previously infected people are better protected when they encounter the virus again than vaccinated people who encounter it the first time 4, 6, 41, 71, 77, 81, 119, 127, 135. Infection provides effective 118, 127, 130, 153, 154 and longer-lasting protection against both primary strains and variants than vaccines do, potentially lifelong protection and long enough to ensure mild or symptom-free infection the following year.
It should be remembered that infection does not only occur from contact and droplets (from a distance of 7-8 meters / 23-26 feet) from coughing or sneezing. The virus particles are so small that they are airborne, so-called aerosols, and float around the air indoors for hours199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, e.g. in a supermarket. They have a size of only approx. 0.002 mm in diameter 200, 205 and therefore masking or distancing doesn’t stop them from entering your body. As such, we are exposed to viruses whether we want to or not.
The common cold provides non-specific protection (cross-immunity) against Covid symptoms 13, 20, 25, 36, 52, 86, 87, 89, 91, 99, 102, 103, 120, 141, 159, 166, 167, 170, 175, 177, 179, 180, 184, 187, 189, 194, 197.
Eighty to ninety percent 87, 116 of the general population are fully or partially immune without previous SARS-CoV-2 infection, even if testing negative for antibodies 86, 107, 139, 144, 151, 170, 189, 192. Studies show that vaccination can impact the immune system negatively 6, 8, 53, 111, 221, 222, 223, 224, 230.
Overall, the studies show that natural infection immunity is broader, stronger, and longer-lasting than artificial vaccine immunity, because the memory cells mature correctly and optimally during and after infection 22, 36, 47, 62, 105, 130, 192, and therefore activate antibodies better and faster 84, 238 than after vaccination 120, 130, 150, 161, 192, 197.
We are constantly infected and immunized, the virus mutates, we are then infected with the variant and get no or only mild symptoms, depending on the individual nutritional health 215, 217, 244 and the degree of mutation of the virus. It is important for the physical resilience of the population that the healthy individuals are immunized naturally, whereby the weak individuals are automatically protected as well.
If we interrupt the continuous exposure to infection by distancing, we will be at risk when society reopens, because we will suddenly and abruptly encounter many new variants, to which we have no immunity.
The Elderly
Studies show a greater risk of variant infection for one month after vaccination in those vaccinated, especially in nursing home residents and addicts with lifestyle diseases17.
Instead of a one-sided focus on vaccines, prevention should also include prevention of immune weakening, which can often be solved easily and at a low cost.
A study found that immunity in the elderly against recognized viruses is good, but worse against new ones 90. This suggests that the elderly should be in mutual contact for as long as possible throughout their lives, as a wide range of viruses continuously stimulate their immune system. It also speaks against physical distancing, since avoiding the virus in one season will result in it hitting harder the following year. This was clearly seen in the mortality trajectory in Denmark and Sweden in 2020-2021 and in Australia this year.
Sweden
The chief epidemiologist of Sweden, A. Tegnell, assessed the danger of Covid to be low, and his advice to the politicians to not shut down Sweden ensured rapid infection immunity in the population.
Sweden coped well with the pandemic. This despite having 15% more >80-year-olds229 and twice as many dark-skinned immigrants 225, 226 (with a reduced ability to produce enough vitamin D in our latitudes) as in Denmark.
Sweden’s relative number of deaths with Covid-19 in 2020-21 matches, without lockdowns, that of Denmark 227.
Vitamin D
It is well-documented that vitamin D is necessary for normal infection processes. It is easy and cheap to remedy the deficiency and thus immune weakening of the elderly and dark-skinned people. This was demonstrated through 60 years of international research. In a 2016 public television documentary it was estimated that vitamin D deficiency costs Denmark 30 billion DKK annually 228 (= 4 billion USD)
Antiviral vitamins and minerals do not cure or prevent Covid, but they can normalize, relieve, and shorten the illness 244, 250. Deficiencies are potentially dangerous and should be eliminated as they are widespread, even in Denmark.
A new large meta-analysis 215 shows that a 3-fold increase in vitamin D in the blood compared to Danish recommendations will lower the already low Covid mortality to, theoretically, zero.
A Superweapon?
Instead of blindly trusting vaccines as “superweapons”, we should remember that we have an effective, natural immune defense when the body is otherwise functioning normally 217. In future epidemics, immunodeficiency due to vitamin deficiency should be minimized. Immunity is more than infection and vaccines 61, 250. Our nutritional health and lifestyle also affect our resilience.
Simple cheap prevention works not only on immunity but is broadly preventative against a number of diseases and must be promoted with campaigns.
Furthermore, people’s own possibilities for natural prevention should never again 134, 236 be neglected or obstructed.
Finally, socially harmful distancing policies and lockdowns should be avoided, unless a pandemic of an extremely dangerous disease occurs, which neither Covid nor Influenza is.
* * * * *
Conflicts of interest: None.
Literature- and source overview:
1) TjekDet.dk: Lever uvaccinerede unge livet farligt? Det mener Søren Brostrøm, men forskere maner til besindighed
(Do unvaccinated young people lead dangerous lives? Søren Brostrøm thinks so, but researchers urge caution.)
https://www.tjekdet.dk/indsigt/lever-uvaccinerede-unge-livet-farligt-det-mener-soeren-brostroem-men-forskere-maner-til
2) Pilz et al: SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environmental Research, 2022, Feb. 2022 Jun; 209: 112911.
”Risk of hospitalizations and deaths was also reduced in SARS-CoV-2 reinfections versus primary infections. Observational studies indicate that natural immunity may offer equal or greater protection against SARS-CoV-2 infections compared to individuals receiving two doses of an mRNA vaccine, but data are not fully consistent. The combination of a previous SARS-CoV-2 infection and a respective vaccination, termed hybrid immunity, seems to confer the greatest protection against SARS-CoV-2 infections, but several knowledge gaps remain regarding this issue. Natural immunity should be considered for public health policy regarding SARS-CoV-2”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8824301/
3) Alejo et al: Prevalence and Durability of SARS-CoV-2 Antibodies Among Unvaccinated US Adults by History of COVID-19. JAMA, 2022, March, 15;327(11):1085-1087.
“Although evidence of natural immunity in unvaccinated healthy US adults up to 20 months after confirmed COVID-19 infection is encouraging, it is unclear how these antibody levels correlate with protection against future SARS-CoV-2 infections”.
https://jamanetwork.com/journals/jama/fullarticle/2788894
https://pubmed.ncbi.nlm.nih.gov/35113143/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8814952/
4) Cohen et al: Long-term humoral immunity of COVID-19 recovered and BNT162b2 vaccinated individuals: a prospective comparative study. European Congress of Clinical Microbiology & Infectious Diseases (ECCMID 2022, Lisbon, 23-26 April)
“While the quantity of antibodies decrease with time in both COVID-19 recovered patients and vaccinated individuals, the quality of antibodies increases following infection but not after vaccination. Obese individuals have a significantly higher and sustained humoral response following infection. These results provide specific characteristics of the immune response that may explain the differential protection against COVID-19 in previously infected and vaccinated individuals”.
https://drive.google.com/file/d/17Hjiz-d8JXsbTdVpmHQNpmKn_jvSWXIf/view
https://www.eurekalert.org/news-releases/942946
5) Willyard et al: What the Omicron wave is revealing about human immunity. Nature, 602, 22-25 (2022)
”A drop in antibody levels after infection is normal. What immunologists really want to know is where – or whether – the decline will stop. In April 2020, Ahmed and his team began studying people who had recovered from COVID-19. The scientists found that those people’s antibody levels dropped quickly for the first two or three months after infection. But then, after about four months, the researchers saw the curve start to flatten. They have published results on the first eight months, but now have data up to 450 days, and Ahmed is encouraged by what they see. So far, “looking at the shape of the curve, it looks pretty damn good”, he says. “It is really quite stable”.
https://www.nature.com/articles/d41586-022-00214-3
6) Kojima et al: Protective immunity after recovery from SARS-CoV-2 infection. The Lancet Infectious Disesases, 2022 Jan; 22(1): 12–14.
“Some people who have recovered from COVID-19 might not benefit from COVID-19 vaccination. In fact, one study found that previous COVID-19 was associated with increased adverse events following vaccination with the Comirnaty BNT162b2 mRNA vaccine (Pfizer–BioNTech). In addition, there are rare reports of serious adverse events following COVID-19 vaccination. In Switzerland, residents who can prove they have recovered from a SARS-CoV-2 infection through a positive PCR or other test in the past 12 months are considered equally protected as those who have been fully vaccinated.
Although longer follow-up studies are needed, clinicians should remain optimistic regarding the protective effect of recovery from previous infection. Community immunity to control the SARS-CoV-2 epidemic can be reached with the acquired immunity due to either previous infection or vaccination… Given the evidence of immunity from previous SARS-CoV-2 infection, however, policy makers should consider recovery from previous SARS-CoV-2 infection equal to immunity from vaccination for purposes related to entry to public events, businesses, and the workplace, or travel requirements.
Researchers have also found that people who recovered from SARS-CoV infection in 2002–03 continue to have memory T cells that are reactive to SARS-CoV proteins 17 years after that outbreak. Additionally, a memory B-cell response to SARS-CoV-2 evolves between 1·3 and 6·2 months after infection, which is consistent with longer-term protection.
We reviewed studies published in PubMed from inception to September 28, 2021 and found well-conducted biological studies that demonstrated protective immunity after infection. Furthermore, multiple epidemiological and clinical studies, including studies during the recent period of predominantly delta (B.1.617.2) variant transmission, found that the risk of repeat SARS-CoV-2 infection decreased by 80·5–100% among those who had had COVID-19 previously (panel). The reported studies were large and conducted throughout the world. Another laboratory-based study that analysed the test results of 9119 people with previous COVID-19 from Dec 1, 2019, to Nov 13, 2020, found that only 0·7% became reinfected”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8575467/
7) Qureshi et al.: Reinfection With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Patients Undergoing Serial Laboratory Testing. Clinical Infectious Diseases, 2022 Jan 15; 74(2): 294–300.
“We identified a low rate of reinfection confirmed by laboratory tests in a large cohort of patients with SARS-CoV-2 infection. Although reinfection appeared to be milder than primary infection, there was associated mortality… Reinfection was identified in 0.7% (n = 63, 95% CI: .5%–.9%) of the patients…
Due to concerns for reinfection, the Centers for Disease Control and Prevention [54] currently recommends vaccination for patients who had SARS-CoV-2 infection after 90 days but acknowledges that limited data available to support the recommendation”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8135382/
8) Seneff and McColluch et al.: Innate Immune Suppression by SARS-CoV-2 mRNA Vaccinations: The role of G-quadruplexes, exosomes and microRNAs. Food and Chemical Toxicology, 2022 Jun;164:113008.
“The mRNA SARS-CoV-2 vaccines were brought to market in response to the widely perceived public health crises of Covid-19. The utilization of mRNA vaccines in the context of infectious disease had no precedent, but desperate times seemed to call for desperate measures. The mRNA vaccines utilize genetically modified mRNA encoding spike proteins. These alterations hide the mRNA from cellular defenses, promote a longer biological half-life for the proteins, and provoke higher overall spike protein production. However, both experimental and observational evidence reveals a very different immune response to the vaccines compared to the response to infection with SARS-CoV-2. As we will show, the genetic modifications introduced by the vaccine are likely the source of these differential responses. In this paper, we present the evidence that vaccination, unlike natural infection, induces a profound impairment in type I interferon signaling, which has diverse adverse consequences to human health. We explain the mechanism by which immune cells release into the circulation large quantities of exosomes containing spike protein along with critical microRNAs that induce a signaling response in recipient cells at distant sites. We also identify potential profound disturbances in regulatory control of protein synthesis and cancer surveillance. These disturbances are shown to have a potentially direct causal link to neurodegenerative disease, myocarditis, immune thrombocytopenia, Bell’s palsy, liver disease, impaired adaptive immunity, increased tumorigenesis, and DNA damage. We show evidence from adverse event reports in the VAERS database supporting our hypothesis. We believe a comprehensive risk/benefit assessment of the mRNA vaccines excludes them as positive contributors to public health, even in the context of the Covid-19 pandemic”.
https://pubmed.ncbi.nlm.nih.gov/35436552/
9) Hancke et al.: Analyse af Covid-19 situationen (Analysis of the Covid-19 situation), Vitalrådet, 2022 Jan.
”In terms of danger compared to flu, there is not much difference. The Delta variant seems to be like a severe flu for a few percent where it settles in the lower respiratory tract. It does not infect a large part of the population, but has roughly the same mortality rate as influenza.
The Omikron variant spreads significantly faster than the delta variant and influenza with a doubling time of 1.2 days. On the other hand, it is significantly milder, settles mainly only in the upper respiratory tract and has meant a large decrease in the need for hospitalization and intensive treatment, just as mortality is very low, almost insignificant.
There seems to be a fundamental biological misconception behind the development of vaccines if the idea was that they should be “a superweapon” to stop an epidemic, let alone a pandemic.
The vaccines do not protect against infection or re-infection, but provide a declining protection against serious illness and death for just over 3 months.
But after 3-4 months, the vaccine effect is directly negative for Omikron, so that the risk of becoming infected is 76% greater than if you have not been vaccinated at all. In terms of infection, the vaccines have no effect on the Omikron variant, which removes any argument for vaccinating children. Furthermore, the available data show that reinfection occurs mainly in vaccinated and not in persons with natural immunity after Covid-19”.
https://www.vitalraadet.dk/da/analyse-af-covid-19-situationen/
10) Altarawneh et al.: Protection afforded by prior infection against SARS-CoV-2 reinfection with the Omicron variant. New England Journal of Medicine, 2022 March 2022; 386:1288-1290.
“PES (prior infection in preventing reinfection) against symptomatic reinfection was estimated at 90.2% (95% CI: 60.2-97.6) for Alpha, 84.8% (95% CI: 74.5-91.0) for Beta, 92.0% (95% CI: 87.9-94.7) for Delta, and 56.0% (95% CI: 50.6-60.9) for Omicron. None progressed to critical or fatal COVID-19. Protection afforded by prior infection in preventing symptomatic reinfection with Alpha, Beta, or Delta is robust, at about 90%. While such protection against reinfection with Omicron is lower, it is still considerable at nearly 60%. Prior-infection protection against hospitalization or death at reinfection appears robust, regardless of variant”.
https://www.medrxiv.org/content/10.1101/2022.01.05.22268782v1
New England Journal of Medicine:
“Overall, in a national database study in Qatar, we found that the effectiveness of previous infection in preventing reinfection with the alpha, beta, and delta variants of SARS-CoV-2 was robust (at approximately 90%), findings that confirmed earlier estimates. Such protection against reinfection with the omicron variant was lower (approximately 60%) but still considerable. In addition, the protection of previous infection against hospitalization or death caused by reinfection appeared to be robust, regardless of variant.
https://www.nejm.org/doi/full/10.1056/nejmc2200133
11) Chivese et al.: The prevalence of adaptive immunity to COVID-19 and reinfection after recovery – a comprehensive systematic review and meta-analysis, Pathogens and Global Health, 2022 Jul;116(5):269-281
“Fifty-four studies from 18 countries, with around 12,000,000 individuals, followed up to 8 months after recovery, were included. Around 90% of recovered individuals had evidence of immunological memory to SARS-CoV-2, at 6-8 months after recovery and had a low risk of reinfection”.
https://pubmed.ncbi.nlm.nih.gov/35099367/
12) A. Sigal: Milder disease with Omicron: is it the virus or the pre-existing immunity? Nature reviews Immunology, 2022 Jan, pages 69–71 (2022)
”Is Omicron infection really milder than Delta, or have the populations that Omicron is infecting built up enough immunity so that the disease course will be milder with any variant of SARS-CoV-2? There is support for both scenarios”.
https://www.nature.com/articles/s41577-022-00678-4
13) Kundu et al.: Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts, Nature Communications, 2022 Jan, 2022; 13: 80.
“We observe higher frequencies of cross-reactive (p = 0.0139), and nucleocapsid-specific (p = 0.0355) IL-2-secreting memory T cells in contacts who remained PCR-negative despite exposure…
Our results are thus consistent with pre-existing non-spike cross-reactive memory T cells protecting SARS-CoV-2-naïve contacts from infection…”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8748880/
14) Rahman et al.: COVID-19 reinfections among naturally infected and vaccinated individuals, Scientific Reports volume 12, 2022 Jan, Article number: 1438
“Naturally infected populations were less likely to be reinfected by SARS-CoV-2 than the infection-naïve and vaccinated individuals. Although, reinfected individuals did not suffer severe disease, a remarkable proportion of naturally infected or vaccinated individuals were (re)-infected by the emerging variants”.
(”Naturligt inficerede populationer var mindre tilbøjelige til at blive reinficeret med SARS-CoV-2 end de uden tidligere infektion og vaccinerede individer. Selvom reinficerede individer ikke led af alvorlig sygdom, blev en bemærkelsesværdig andel af naturligt inficerede eller vaccinerede individer (re)inficeret af de nye varianter”.)
https://www.nature.com/articles/s41598-022-05325-5
15) Rivelli et al,: Incidence of COVID-19 reinfection among Midwestern healthcare employees, PLOS ONE, 2022 Jan., doi: 10.1371/journal.pone.0262164
“This study supports the consensus that COVID-19 reinfection, defined as subsequent infection ≥ 90 days after prior infection, is rare, even among a sample of healthcare workers with frequent exposure”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8726474/
16) León et al.: COVID-19 Cases and Hospitalizations by COVID-19 Vaccination Status and Previous COVID-19 Diagnosis – California and New York, May-November 2021. Morbidity and Mortality Weekly Report (MMWR), 2022 Jan 28;71(4):125-131.
“Importantly, infection-derived protection was higher after the Delta variant became predominant, a time when vaccine-induced immunity for many persons declined because of immune evasion and immunologic waning”.
https://pubmed.ncbi.nlm.nih.gov/35085222/
17) Hollinghurst et al.:
COVID-19 infection risk amongst 14,104 vaccinated care home residents: a national observational longitudinal cohort study in Wales, UK, December 2020–March 2021. Age and Ageing, 2022 Jan; 51(1): afab223.
”COVID-19 infection risk amongst 14,104 vaccinated care home residents… Increased risk of infection after 21 days was associated with frailty. We found most infections occurred within 28 days of vaccination, suggesting extra precautions to reduce transmission risk should be taken in this time frame”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8690013/
18) Spizer et al.: Protective Immunity after Natural Infection with Severe Acute Respiratory Syndrome Corona-virus-2 (SARS-CoV-2) – Kentucky, USA, 2020. International Journal of Infectious Diseases, 2022 Jan; 114: 21–28.
“Natural infection provides substantial and persistent immunologic protection for a period of several months for most individuals”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8506664/
19) Mobaraki et al.: Long-Term Persistence of IgG Antibodies in recovered COVID-19 individuals at 18 months and the impact of two-dose BNT162b2 (Pfizer-BioNTech) mRNA vaccination on the antibody response. MedRxiv, 2022.01.18.22269349 (pre-print)
“At 18 months, 97% participants tested positive for anti-NCP (anti-nucleocapsid protein) hinting towards the persistence of infection-induced immunity even for the vaccinated individuals. Our study findings demonstrate that while double dose vaccination boosted the IgG titers in recovered individuals 161 times, this “boost” was relatively short-lived. The unvaccinated recovered individuals, in contrast, continued to show a steady decline but detectable antibody levels”.
https://www.medrxiv.org/content/10.1101/2022.01.18.22269349v1
20) Wang et al.: Exposure to SARS-CoV-2 generates T-cell memory in the absence of a detectable viral infection. Nature Communications, 2021 Mar; 12: 1724
“In summary, by examining a substantial number of clinical samples, we determined the SARS-CoV-2-specific memory T-cell immunity in COVID-19 patients with various clinical symptoms. Despite some subtle differences, most patients developed measurable amounts of SARS-CoV-2-specific CD4+ and CD8+ memory T cells which were stably maintained between 48–86 days after convalescence. Importantly, our discovery of the presence of significant levels of SARS-CoV-2-specific memory T-cell immunity in a group of individuals (close contacts) who were exposed to but not infected by the virus highlights some unique characteristics in the dynamic interactions between SARS-CoV-2 and its human host. Although cross-reactive memory T cells were present in healthy donors who had never been exposed to SARS-CoV-2, their role in host protection needs to be thoroughly investigated as they were hardly able to proliferate”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7979809/#MOESM3
21) Laidlaw et al.: The germinal centre B cell response to SARS-CoV-2. Nature Reviews Immunology, 2022; 22(1): 7–18
“One central question is whether additional ‘booster’ vaccines expressing mRNA from variant strains will be necessary to induce a B cell response with sufficient breadth and affinity to neutralize future SARS-CoV-2 variants. While the administration of a third vaccine dose of the same formulation will likely result in an increase in antibody titres, it is unlikely to profoundly alter the specificity of the memory B cell response…
SARS-CoV-2 vaccines are administered intramuscularly and therefore are unlikely to induce sufficient levels of antigen expression or inflammation in mucosal tissues to support a local GC response. In the absence of a mucosal B cell response, protection from reinfection will be reliant on maintaining a high enough titre of circulating antibodies to neutralize viruses that infect the airways…
Relatedly, understanding how the SARS-CoV-2-specific IgA response differs between vaccinated and infected individuals will be important going forwards. The serum IgA response rapidly declines following both SARS-CoV-2 vaccination and SARS-CoV-2 infection and is less potent at neutralizing SARS-CoV-2 than IgG…
However, SARS-CoV-2 infection also elicits a virus-specific IgG, IgA and IgE antibody response in the saliva and bronchoalveolar fluid. Dimeric SARS-CoV-2-specific IgA, the primary form of IgA present in the nasopharynx, has an enhanced ability to neutralize the virus compared with IgG and may have an important role in preventing reinfection. While it is not known whether SARS-CoV-2 vaccination induces a mucosal IgA response in humans, intramuscular vaccination of mice drove a minimal mucosal IgA response and was not as good at mediating viral clearance at mucosal sites as intranasal vaccination”.
https://www.nature.com/articles/s41577-021-00657-1
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8647067/
22) Lindsley et al.: Understanding memory B cell selection. Journal of Theoretical Biology. 2021 Dec 21; 531:110905
“A core aspect of the adaptive immune system’s humoral response is training two types of B cells through a process called affinity maturation (AM): plasma B cells which generate antibodies to identify the current antigen, and memory B cells which are used in subsequent immune responses to identify similar antigens in the future. The AM process is highly unusual, in that a specific region of DNA within participating B cells is mutated to generate offspring which are selected to have higher affinity to the antigen in question. The preservation of DNA sequences is usually of utmost importance in most cells, but the region of the genome which defines the shape of the B cell receptor must be rapidly modified for the B cell receptor to have a chance of becoming better at recognizing the antigen of interest (Meyer-Hermann et al., 2012). These mutations are responsible for the B cells’ incredible ability to recognize practically any antigen that they are presented, making the mammalian adaptive immune system one of the most effective learned identification systems in the natural world”.
https://www.sciencedirect.com/science/article/pii/S0022519321003246?via%3Dihub
https://pubmed.ncbi.nlm.nih.gov/34543633/
23) Goldberg et al.: Protection and waning of natural and hybrid COVID-19 immunity. The New England Journal of Medicine, 2022 Jun 9;386(23):2201-2212.
“Protection from reinfection decreases with time since previous infection, but is, nevertheless, higher than that conferred by vaccination with two doses at a similar time since the last immunity-conferring event.”
https://www.medrxiv.org/content/10.1101/2021.12.04.21267114v1
https://pubmed.ncbi.nlm.nih.gov/35613036/
24) Dowell et al.:
Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nature Immunology, 2021 Dec., Nature Immunology; 23(1): 40–49.
“Importantly, children retained antibody and cellular responses 6 months after infection, whereas relative waning occurred in adults. Spike-specific responses were also broadly stable beyond 12 months. Therefore, children generate robust, cross-reactive and sustained immune responses to SARS-CoV-2 with focused specificity for the spike protein. These findings provide insight into the relative clinical protection that occurs in most children and might help to guide the design of pediatric vaccination regimens”.
https://www.nature.com/articles/s41590-021-01089-8
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8709786/
25) Milne et al.: Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? The Lancet Respiratory Medicine, 2021 Dec; 9(12): 1450–1466
“SARS-CoV-2 infection elicits an adaptive immune response against a large breadth of viral epitopes, although the duration of the response varies with age and disease severity… Current evidence from case studies and large observational studies suggests that, consistent with research on other common respiratory viruses, a protective immunological response lasts for approximately 5–12 months from primary infection”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8530467/
26) Adamo et al.: Signature of long-lived memory CD8+ T cells in acute SARS-CoV-2 infection. Nature, 2022; 602(7895): 148–155.
“Here, using spectral flow cytometry combined with cellular indexing of transcriptomes and T cell receptor sequencing, we longitudinally characterized individual SARS-CoV-2-specific CD8+ T cells of patients with COVID-19 from acute infection to 1 year into recovery and found a distinct signature identifying long-lived memory CD8+ T cells… Collectively, we describe a transcriptional signature that marks long-lived, circulating human memory CD8+ T cells following an acute viral infection”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8810382/
27) Saade et al.: Live virus neutralization testing in convalescent patients and subjects vaccinated against 19A, 20B, 20I/501Y.V1 and 20H/501Y.V2 isolates of SARS-CoV-2. Emerging Microbes & Infections, 2021; 10(1): 1499–1502.
“However, a significant decrease in neutralization ability was found for 20I/501Y.V1 in comparison with 19A isolate for critical patients and HCWs 6-months post infection. Concerning 20H/501Y.V2, all populations had a significant reduction in neutralizing antibody titers in comparison with the 19A isolate. Interestingly, a significant difference in neutralization capacity was observed for vaccinated HCWs between the two variants but not in the convalescent groups”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8330769/
28) A. Israel et al.: Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection. Vaccines (Basel), 2022 Jan; 10(1): 64.
“This study demonstrates individuals who received the Pfizer-BioNTech mRNA vaccine have different kinetics of antibody levels compared to patients who had been infected with the SARS-CoV-2 virus, with higher initial levels but a much faster exponential decrease in the first group”. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8781423/
29) Almendro-Vázquez et al.: Longitudinal dynamics of SARS-CoV-2-specific cellular and humoral immunity after natural infection or BNT162b2 vaccination. PLOS Pathogens, 2021 Dec; 17(12): e1010211
”Three months post-vaccination, the cellular response was comparable, while the humoral response was consistently stronger, to that measured in COVID-19 recovered patients. Thus, measurement of both humoral and cellular responses provides information on prognosis and protection from infection, which may add value for individual and public health recommendations”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8757952/
30) S. Sureshchandra et al.: Single-cell profiling of T and B cell repertoires following SARS-CoV-2 mRNA vaccine. JCI Insight, 2021 Dec 22; 6(24): e153201
”Natural infection induced expansion of larger CD8 T cell clones occupied distinct clusters, likely due to the recognition of a broader set of viral epitopes presented by the virus not seen in the mRNA vaccine. Our study highlights a coordinated adaptive immune response where early CD4 T cell responses facilitate the development of the B cell response and substantial expansion of effector CD8 T cells, together capable of contributing to future recall responses”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8783687/
31) A. Haveri et al.: Persistence of neutralizing antibodies a year after SARS‐CoV‐2 infection in humans. European Journal of Immunology, 2021 Dec; 51(12): 3202–3213.
“We found that NAb (Neutralizing Anti bodies, red.) against the WT (Wild Type) virus persisted in 89% and S-IgG in 97% of subjects for at least 13 months after infection”. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8646652/
32) Keeton et al.: SARS-CoV-2 spike T cell responses induced upon vaccination or infection remain robust against Omicron. Nature, 2022; 604(7907): E25.
“These results demonstrate that despite Omicron’s extensive mutations and reduced susceptibility to neutralizing antibodies, the majority of T cell response, induced by vaccination or natural infection, cross-recognises the variant. Well-preserved T cell immunity to Omicron is likely to contribute to protection from severe COVID-19, supporting early clinical observations from South Africa.”
“Overall, our data show that unlike neutralizing antibodies, the SARS-CoV-2 T cell responses generated upon vaccination or previous infection are highly cross-reactive with Omicron”.
https://www.medrxiv.org/content/10.1101/2021.12.26.21268380v1
https://pubmed.ncbi.nlm.nih.gov/35102311/
https://www.nature.com/articles/s41586-022-04460-3
33) Chemaitelly et al.: Efficacy of Natural Immunity against SARS-CoV-2 Reinfection with the Beta Variant. New England journal of Medicine, 2021 Dec 15 : NEJMc2110300.
”Protection by previous SARS-CoV-2 infection against reinfection with the beta variant was observed, even 1 year after the primary infection, but protection was slightly lower than that against the alpha variant and wild-type virus circulating in Qatar.3-5 These findings give some insights into the hypothesis that natural immunity may provide protection against known variants of concern”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8693689/
34) Z. Lyski et al.: SARS-CoV-2 specific memory B-cells from individuals with diverse disease severities recognize SARS-CoV-2 variants of concern. Journal of Infectious Diseases, 2022 Mar 15;225(6):947-956.
“This finding, that VoC-RBD-reactive MBCs are present in the peripheral blood of all subjects including those that experienced asymptomatic or mild disease, provides a reason for optimism regarding the capacity of vaccination, prior infection, and/or both, to limit disease severity and transmission of variants of concern as they continue to arise and circulate”.
https://pubmed.ncbi.nlm.nih.gov/34865053/
35) N. Kojima et al.: A Systematic Review of the Protective Effect of Prior SARS-CoV-2 Infection on Repeat Infection. Evaluation and The Health Professions, 2021 Dec; 44(4): 327–332.
“The protective effect of prior SARS-CoV-2 infection on re-infection is high and similar to the protective effect of vaccination”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8564250/
36) Ortega et al.: Seven-month kinetics of SARS-CoV-2 antibodies and role of pre-existing antibodies to human coronaviruses. Nature Communications, 2021 Aug.; 12: 4740.
“Impact of pre-existing antibodies to human coronaviruses causing common cold (HCoVs), is essential to understand protective immunity to COVID-19… after the peak response, anti-spike antibody levels increase from ~150 days post-symptom onset in all individuals (73% for IgG), in the absence of any evidence of re-exp
Thus, pre-existing cross-reactive HCoVs antibodies could have a protective effect against SARS-CoV-2 infection and COVID-19 disease.,, Strong correlations were found between antibody neutralization capacity and the days PSO, as identified in the previous literature…, in accordance with the antibody affinity increase after the maturation of the immune response”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8346582/
37) S. Mahajan et al.: Immunodominant T-cell epitopes from the SARS-CoV-2 spike antigen reveal robust pre-existing T-cell immunity in unexposed individuals. Scientific Reports, 2021 June; 11: 13164.
“Our findings suggest that SARS-CoV-2 reactive T-cells are likely to be present in many individuals because of prior exposure to flu and CMV viruses (Cytomegalovirus, red.)”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8222233/
38) B. Mizrahi et al.: Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nature Communications, 2021 Nov; 12: 6379.
“After controlling for potential confounders as age and comorbidities, we found a significant 1.51 fold (95% CI, 1.38–1.66) increased risk for infection for early vaccinees compared to those vaccinated later that was similar across all ages groups. The increased risk reached 2.26- fold (95% CI, 1.80–3.01) when comparing those who were vaccinated in January to those vaccinated in April. This preliminary finding of vaccine waning as a factor of time from vaccine should prompt further investigations into long-term protection against different strains”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8569006/
39) S. Andeweg et al.: Increased risk of infection with SARS-CoV-2 Beta, Gamma, and Delta variant compared to Alpha variant in vaccinated individuals. Science Translational Medicine, 2022 Jul 21; eabn4338
”I modsætning til vaccine-induceret immunitet blev der ikke fundet nogen øget risiko for reinfektion med Beta-, Gamma- eller Delta-varianter i forhold til Alpha-varianten hos individer med infektionsinduceret immunitet”.
https://www.medrxiv.org/content/10.1101/2021.11.24.21266735v1.full-text
https://pubmed.ncbi.nlm.nih.gov/35862508/
40) L. Abu-Raddad et al.: Severity of SARS-CoV-2 Reinfections as Compared with Primary Infections. New England Journal of Medicine, 2021 Nov 24: NEJMc2108120.
“Reinfections had 90% lower odds of resulting in hospitalization or death than primary infections. Four reinfections were severe enough to lead to acute care hospitalization. None led to hospitalization in an ICU, and none ended in death. Reinfections were rare and were generally mild, perhaps because of the primed immune system after primary infection”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8631440/
41) R. Satwik et al.: ChAdOx1 nCoV-19 effectiveness during an unprecedented surge in SARS COV-2 infections.
European Journal of Internal Medicine, 2021 Nov; 93: 112–113.
“The third key finding is that previous infections with SARS-CoV-2 were significantly protective against all studied outcomes, with an effectiveness of 93% (87 to 96%) seen against symptomatic infections, 89% (57 to 97%) against moderate to severe disease and 85% (-9 to 98%) against supplemental oxygen therapy. All deaths occurred in previously uninfected individuals. This was higher protection than that offered by single or double dose vaccine”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8364816/
42) B. Sciscent et al.: COVID-19 reinfection: the role of natural immunity, vaccines, and variants. Journal of Community Hospital Internal Medicine Perspectives, 2021; 11(6): 733–739.
“The definition of reinfection has been interpreted differently across many studies. The Centers for Disease Control defined reinfection as an infection in the same individual across a different time period with evidence of genotypic variance, i.e., infection in an individual with two different viral strains within ≥45 days in highly suspicious cases of COVID-19 or ≥90 days in asymptomatic cases or in cases with low suspicion[3].
The above model also takes into consideration cycle threshold values less than or equal to 35[9]. Many other studies define reinfection as two positive SARS-CoV-2 RT-PCR tests with negative tests in between without taking the genotypic variation into account.
Hall et al. reported that the prior history of SARS-CoV-2 is associated with an 83% lower risk of reinfection and that the protective effect may last for 5 months[10]. In a large population study done in Denmark by Hansen et al., protection against repeat infection was deemed to be 80.5% in the general population and 47.1% in patients 65 years or older[11].
However, this study defined reinfection differently. Here, the authors included people who were tested with COVID-19 RT-PCR during the first surge before June 2020 and followed the cohort from September to December 2020 to analyze SARS-CoV-2 contraction[11].
Abu-Raddad et al. studied the efficacy of natural infection against reinfection, which was accounted for by a change in viral genome sequencing. This study found that the rate of reinfection was estimated to be 95.2%”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8604456/
43) A. Jeffrey-Smith et al.: SARS-CoV-2–specific memory B cells can persist in the elderly who have lost detectable neutralizing antibodies. The Journal of Clinical Investigation, 2022 Jan 18;132(2):e152042
“Our findings demonstrate that a reserve of SARS-CoV-2–specific MBCs persists beyond the loss of nAbs…
In conclusion, by focusing on an elderly cohort with a high proportion of nAb loss, we demonstrated that this waning in the first line of humoral defense could be compensated by the presence of a reserve of adaptive B cell memory in the majority of cases. Our findings highlight the importance of including measures of B cell memory in larger studies of natural infection and vaccination to determine their role as additional correlates of protection.
Our data underscore the idea that identifying antigen-specific B cells by tetramer antigen staining is useful for quantitation and thorough ex vivo characterization, but may not necessarily equate with the preservation of a functional response, in line with discrepancies between the frequency and function of MBCs described in chronic viral infection).
The relative preservation of IgA antigen–specific MBCs in those with waned serum nAb raises the possibility that mucosal sequestered immunity may outlast that which is detectable in the circulation. Increased expansion of activated MBCs in the elderly highlights the need to investigate whether these cells are more prone to prolonged stimulation from persistent reservoirs of SARS-CoV-2 antigen.”
https://www.jci.org/articles/view/152042
https://pubmed.ncbi.nlm.nih.gov/34843448/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8759779/
44) Kojima et al.: A Systematic Review of the Protective Effect of Prior SARS-CoV-2 Infection on Repeat Infection. Evaluation and The Health Professions 2021 Dec; 44(4): 327–332.
“We identified 1,392 reports. Of those, 10 studies were eligible for our systematic review. The weighted average risk reduction against reinfection was 90.4% with a standard deviation of 7.7% (p-value: <0.01). Protection against SARS-CoV-2 reinfection was observed for up to 10 months. Studies had potential information, selection, and analysis biases. The protective effect of prior SARS-CoV-2 infection on re-infection is high and similar to the protective effect of vaccination”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8564250/
45) Sarraf et al.: Immunity to COVID-19 in India through vaccination and natural infection. MedRxiv (pre-print), https://doi.org/10.1101/2021.11.08.21266055
“We compared the vaccination induced immune response profile with that of natural infection, evaluating thereby if individuals infected during the first wave retained virus specific immunity…
The overall immune response resulting from natural infection in and around Kolkata is not only to a certain degree better than that generated by vaccination, especially in the case of the Delta variant, but cell mediated immunity to SARS-CoV-2 also lasts for at least ten months after the viral infection”.
https://www.medrxiv.org/content/10.1101/2021.11.08.21266055v1
46) Abu-Raddah et al.: Assessment of the Risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Reinfection in an Intense Reexposure Setting. Clinical Infectious Diseases, 2021 Oct 1; 73(7): e1830–e1840.
“SARS-CoV-2 reinfection can occur but is a rare phenomenon suggestive of protective immunity against reinfection that lasts for at least a few months post primary infection”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7799253/
47) L. de Campos‐Mata et al.: SARS‐CoV‐2 sculpts the immune system to induce sustained virus‐specific naïve‐like and memory B‐cell responses. Clinical & Translational Immunology, 2021; 10(9): e1339.
”SARS‐CoV‐2 sculpts the immune system to induce sustained virus‐specific naïve‐like and memory B‐cell responses. In summary, our in‐depth characterisation of SARS‐CoV‐2‐specific B‐cell responses revealed a previously unappreciated expansion of virus‐specific naïve‐like B cells over time, perhaps through the continuous mobilisation of mature B‐cell precursors to the periphery. Moreover, our results consolidated previous findings on the immune response dynamics occurring in COVID‐19 patients, showing both transient and long‐lasting changes associated with disease severity and development of immune memory”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8418925/
48) Y. Mao et al.: Reinfection rates among patients previously infected by SARS-CoV-2: systematic review and meta-analysis. Chinese Medical Journal, 2022 Jan 20; 135(2): 145–152
“The rate of reinfection with SARS-CoV-2 is relatively low. The protection against SARS-CoV-2 after natural infection is comparable to that estimated for vaccine efficacy”. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8769121/
49) Vimvara Vacharathit et al.: CoronaVac induces lower neutralising activity against variants of concern than natural infection. Lancet, Infectious diseases, 2021 Oct; 21(10): 1352–1354.
CoronaVac induces lower neutralising activity against variants of concern than natural infection
“Overall, the percentage of participants with quantifiable NAb titres (≥20 units) was highest against the WT strain, followed by much lower titres against the alpha, beta, and delta variants (appendix p 5). This pattern was consistently observed in all cohorts, and notably, the percentages of individuals with detectable NAbs were lower in CoronaVac recipients than in the naturally infected cohorts “.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8389976/
50) J. Wei et al.: Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nature Communications, 2021; 12: 6250.
“We estimated antibody levels associated with protection against reinfection likely last 1.5-2 years on average, with levels associated with protection from severe infection present for several years”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8556331/
51) J. Vitale et al.: Assessment of SARS-CoV-2 Reinfection 1 Year After Primary Infection in a Population in Lombardy, Italy. JAMA Internal Medicine, 2021 Oct; 181(10): 1407–1408
“The study results suggest that reinfections are rare events and patients who have recovered from COVID-19 have a lower risk of reinfection. Natural immunity to SARS-CoV-2 appears to confer a protective effect for at least a year”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8164145/
52) L. Loyal, Drosten et al.:
Cross-reactive CD4+ T cells enhance SARS-CoV-2 immune responses upon infection and vaccination, Science, 2021 Oct 8;374(6564):eabh1823
”There is mounting evidence that immunological memory after infection with seasonal human coronaviruses (hCoVs) contributes to cross-protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)… Preexisting cross-reactive immunity may be responsible for the unexpectedly rapid induction of protective immunity after primary SARS-CoV-2 immunization and the high rate of asymptomatic and mild COVID-19 disease courses”.
https://www.science.org/doi/10.1126/science.abh1823?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
https://pubmed.ncbi.nlm.nih.gov/34465633/
53) H. Banoun: Covid-19: Natural immunity versus vaccine immunity. Qeios, Oct 1, 2021
“Survivors of the 2003 SARS-CoV have cellular immune memory more than 17 years after infection.
Numerous studies have explored humoral (antibody) and cellular immunity to SARS-CoV-2 after Covid-19 infection: it persists for at least one year (and even 14 months) in a robust manner in convalescents and would be of better quality than vaccine immunity: natural antibodies are more potent, have a broader spectrum, and are able to evolve against variants more efficiently than vaccine antibodies.
These in vitro studies are confirmed by the protection against reinfections conferred by a primary infection, particularly in early and highly vaccinated countries such as Israel and the United Kingdom.
Vaccination of a primo-infected person could also decrease the effectiveness of his natural immunity against future reinfections…
These in vitro observations of antibody and memory cell levels are well confirmed by looking for reinfections in convalescents: these are very rare.
Numerous publications (see for bibliography the recent BMJ article16 ) show that the rate of reinfection is very low (less than 1%) following a first infection with SARS-CoV-2. The vast majority of these reinfections are not reinfections as such since they are asymptomatic: they are simply nasal carriage of virus without systemic infection. This is not surprising for a cold virus; the nose is an immune sanctuary where blood antibodies do not circulate.
Natural immunity to Covid-19 (i.e., obtained after natural infection with the virus) is therefore certainly robust and durable.
Work on post-vaccination immunity is mainly concerned with so-called “neutralizing” antibodies in vitro. The levels of these antibodies may not be a good correlate of protection because studies often find higher levels of antibodies after vaccination than after infection. However, reinfections are much more frequent in vaccinated patients than in convalescents. Protection against Covid-19 could rather depend on immune memory (due to memory T and B cells that persist long after infection) and seems to be of better quality than that conferred by vaccines.
In addition, vaccination of convalescent subjects could be risky: more systemic adverse events are observed in convalescent subjects than in naïve subjects after the first dose of vaccine.
Vaccination may decrease the ability to respond to future variants. It could also have a non- specific effect of remodeling the innate immune response by decreasing the potential response to other viruses or to cancers and by modifying the course of inflammatory and autoimmune diseases”.
https://www.qeios.com/read/DP264J
54) H. Marcotte et al.: Immunity to SARS-CoV-2 up to 15 months after infection. Iscience, 2022 Feb 18; 25(2): 103743.
“SARS-CoV-2-specific memory B and T cells persisted in the majority of patients up to 15 months although a significant decrease in specific T cells, but not B cells, was observed between 6 and 15 months. Antiviral specific immunity, especially memory B cells in COVID-19 convalescent patients, is long-lasting, but some variants of concern may at least partially escape the neutralizing activity of plasma antibodies”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8736281/
55) Shenai et al.: Equivalency of Protection From Natural Immunity in COVID-19 Recovered Versus Fully Vaccinated Persons: A Systematic Review and Pooled Analysis. Cureus, 2021 Oct; 13(10): e19102.
“… our review demonstrates that natural immunity in COVID-recovered individuals is, at least, equivalent to the protection afforded by complete vaccination of COVID-naïve populations. There is a modest and incremental relative benefit to vaccination in COVID-recovered individuals; however, the net benefit is marginal on an absolute basis. Therefore, vaccination of COVID-recovered individuals should be subject to clinical equipoise and individual preference”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8627252/
56) Giorgi et al.: Naturally Acquired SARS-CoV-2 Immunity Persists for Up to 11 Months Following Infection. Journal of Infectious Diseases, 2021 Oct 15; 224(8): 1294–1304
“Our data suggest that immunological memory is acquired in most individuals infected with SARS-CoV-2 and is sustained in a majority of patients for up to 11 months after recovery”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8195007/
57) Zhang et al.: One-year sustained cellular and humoral immunities of COVID-19 convalescents. Clinical infectious Diseases, 2021 Oct 5 : ciab884.
“SARS-CoV-2-specific cellular and humoral immunities are durable at least until one year after disease onset”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8524303/
58) E. Callaway: COVID super-immunity: one of the pandemic’s great puzzles. Nature, 2021 Oct;598(7881):393-394.
“Natural infection triggered antibodies that continued to grow in potency and their breadth against variants for a year after infection, whereas most of those elicited by vaccination seemed to stop changing in the weeks after a second dose. Memory B cells that evolved after infection were also more likely than those from vaccination to make antibodies that block immune-evading variants such as Beta and Delta.”
https://www.nature.com/articles/d41586-021-02795-x
https://pubmed.ncbi.nlm.nih.gov/34650244/
59) A. Cho et al.: Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination, Nature, 2021; 600(7889): 517–522.
”Coronavirus 2 (SARS-CoV-2) infection produces B cell responses that continue to evolve for at least a year. During that time, memory B cells express increasingly broad and potent antibodies that are resistant to mutations found in variants of concern…While individual memory antibodies selected over time by natural infection have greater potency and breadth than antibodies elicited by vaccination, the overall neutralizing potency of plasma is greater following vaccination. These results suggest that boosting (previously uninfected, red.) vaccinated individuals with currently available mRNA vaccines will increase plasma neutralizing activity but may not produce antibodies with equivalent breadth to those obtained by vaccinating convalescent individuals.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8674133/
60) R. Reyes et al.:
SARS-CoV-2 spike-specific memory B cells express markers of durable immunity after non-severe COVID-19 but not after severe disease. BioRxiv, Preprint. 2021 Sep 27.
”SARS-CoV-2 infection elicits a robust B cell response, resulting in the generation of long-lived plasma cells and memory B cells… Collectively, our results suggest that the memory B cell response elicited during non-severe COVID-19 may be of higher quality than the response after severe disease”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8491845/
61) J. Boechat et al: The immune response to SARS-CoV-2 and COVID-19 immunopathology – Current perspectives. Pulmonology, 2021 September-October; 27(5): 423–437.
“Severe COVID-19 appears to be due not only to viral infection but also to a dysregulated immune and inflammatory response. In this paper, the authors review the most recent publications on the immunobiology of SARS-CoV-2, virus interactions with target cells, and host immune responses, and highlight possible associations between deficient innate and acquired immune responses and disease progression and mortality… Much of the data from these first 12 months indicate that actions aimed at controlling the inflammatory response and immune dysregulation will be as important as those targeting the virus and its replication mechanisms”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8040543/
62) Pape et al: High-affinity memory B cells induced by SARS-CoV-2 infection produce more plasmablasts and atypical memory B cells than those primed by mRNA vaccines. Celle Reports, 2021 Oct 12; 37(2): 109823.
“Infection-induced primary MBCs have better antigen-binding capacity and generate more plasmablasts and secondary MBCs of the classical and atypical subsets than do vaccine-induced primary MBCs. Our results suggest that infection-induced primary MBCs have undergone more affinity maturation than vaccine-induced primary MBCs and produce more robust secondary responses”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8463313/
63) Eyran et al: The longitudinal kinetics of antibodies in COVID-19 recovered patients over 14 months. PLOS Pathogens, June 3, 2022.
“We found a significantly faster decay in naïve vaccinees compared to recovered patients suggesting that the serological memory following natural infection is more robust compared to vaccination. Our data highlights the differences between serological memory induced by natural infection vs. vaccination, facilitating the decision making in Israel regarding the 3rd dose vaccination”.
(a)
An exploration of the differences in the longitudinal kinetics between recovered patients and naïve vaccinees who had received two doses of the BNT162b2 vaccine showed a significantly faster decay in the naïve vaccinees, indi-cating that serological memory following natural infection is more robust than that following to vaccination”. (b)
(a) https://www.medrxiv.org/content/10.1101/2021.09.16.21263693v1
(b) https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1010569
64) Chen et al: Differential antibody dynamics to SARS-CoV-2 infection and vaccination. BioRxiv, https://doi.org/10.1101/2021.09.09.459504 (pre-print)
“Optimal immune responses furnish long-lasting (durable) antibodies protective across dynamically mutating viral variants (broad). To assess robustness of mRNA vaccine-induced immunity…compared antibody durability and breadth after SARS-CoV-2 infection and vaccination…While vaccination delivered robust initial virus-specific antibodies with some cross-variant coverage, pre-variant SARS-CoV-2 infection-induced antibodies, while modest in magnitude, showed highly stable long-term antibody dynamics…Differential antibody durability trajectories favored COVID-19-recovered subjects with dual memory B cell features of greater early antibody somatic mutation and cross-coronavirus reactivity…illuminating an infection-mediated antibody breadth advantage and an anti-SARS-CoV-2 antibody durability-enhancing function conferred by recalled immunity.”
https://www.biorxiv.org/content/10.1101/2021.09.09.459504v1.abstract
65) F. Maghsood et al.: Differential Antibody Response to SARS-CoV-2 Antigens in Recovered and Deceased Iranian COVID-19 Patients. Viral immunology, 2021 Dec;34(10):708-713
“The levels of IgM and IgG specific to N and RBD proteins were detected by ELISA. N- and RBD-specific IgM was higher in deceased patients in comparison with recovered patients, while there was no significant difference in N- and RBD-specific IgG between the two groups. A significant correlation was observed between IgG and IgM titers against RBD and N, in both groups of patients. These results argue against impaired antibody response in deceased COVID-19 patients”.
https://pubmed.ncbi.nlm.nih.gov/34534012/
66) T. Ma et al.: Protracted yet Coordinated Differentiation of Long-Lived SARS-CoV-2-Specific CD8 + T Cells during Convalescence. Journal of immunology, 2021 Sep 1;207(5):1344-1356
“Over the approximately six month period of convalescence monitored, we observed a slow and progressive decrease in the activation state and polyfunctionality of Nuc322-331-specific CD8+ T cells, accompanied by an increase in their lymph node-homing and homeostatic proliferation potential. These results suggest that following a typical case of mild COVID-19, SARS-CoV-2-specific CD8+ T cells not only persist but continuously differentiate in a coordinated fashion well into convalescence into a state characteristic of long-lived, self-renewing memory”.
https://pubmed.ncbi.nlm.nih.gov/34389625/
67) Garrido et al.: Asymptomatic or mild symptomatic SARS-CoV-2 infection elicits durable neutralizing antibody responses in children and adolescents. JCI Insight, 2021 Sep 8; 6(17): e150909
“We evaluated humoral immune responses in 69 children and adolescents with asymptomatic or mild symptomatic SARS-CoV-2 infection. We detected robust IgM, IgG, and IgA antibody responses to a broad array of SARS-CoV-2 antigens at the time of acute infection and 2 and 4 months after acute infection in all participants. Notably, these antibody responses were associated with virus-neutralizing activity that was still detectable 4 months after acute infection in 94% of children. Moreover, antibody responses and neutralizing activity in sera from children and adolescents were comparable or superior to those observed in sera from 24 adults with mild symptomatic infection. Taken together, these findings indicate that children and adolescents with mild or asymptomatic SARS-CoV-2 infection generate robust and durable humoral immune responses that can likely contribute to protection from reinfection”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8492306/
68) Schiffner et al.: Long-Term Course of Humoral and Cellular Immune Responses in Outpatients After SARS-CoV-2 Infection. Frontiers In Public Health, 2021 Sept.; 9: 732787.
“Our data suggest that immunological reaction is acquired in most individuals after natural infection with SARS-CoV-2 and is sustained in the majority of patients for at least 10 months after infection after a mild or moderate disease course”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8502872/
69) Dehgani-Mobaraki et al.: Longitudinal observation of antibody responses for 14 months after SARS-CoV-2 infection. Clinical immunology, 2021 Sep; 230: 108814.
“Patients reporting loss of smell and taste during the clinical course of the disease developed significantly higher
antibody titers. In conclusion, our study findings are consistent with recent studies reporting antibody persistency suggesting that induced SARS-CoV-2 immunity through natural infection, might be very efficacious against re-infection (>90%) and could persist for more than six months. Our study followed up patients up to 14 months demonstrating the presence of anti-S-RBD IgG in 96.8% of recovered COVID-19 subjects”. https://www.sciencedirect.com/science/article/pii/S1521661621001510
https://pubmed.ncbi.nlm.nih.gov/34343708/
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8325385/
70) Gazit et al.: Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. MedRxiv, https://doi.org/10.1101/2021.08.24.21262415 (pre-print)
”Denne undersøgelse viste, at naturlig immunitet giver længerevarende og stærkere beskyttelse mod infektion, symptomatisk sygdom og hospitalsindlæggelse forårsaget af Delta-varianten af SARS-CoV-2 sammenlignet med den BNT162b2-vaccineinducerede immunitet med to doser”. https://www.medrxiv.org/content/10.1101/2021.08.24.21262415v1
71) Wang et al.: Ultrapotent antibodies against diverse and highly transmissible SARS-CoV-2 variants. Science, 2021 Aug 13; 373(6556).
“Our study demonstrates that convalescent subjects previously infected with ancestral variant SARS-CoV-2 produce antibodies that cross-neutralize emerging VOCs with high potency…
We identified four receptor binding domain-targeting antibodies from three early-outbreak convalescent donors with potent neutralizing activity against 23 variants, including the B.1.1.7, B.1.351, P.1, B.1.429, B.1.526, and B.1.617 VOCs”. https://pubmed.ncbi.nlm.nih.gov/34210892/
72) Rank et al.: One Year after Mild COVID-19: The Majority of Patients Maintain Specific Immunity, But One in Four Still Suffer from Long-Term Symptoms. Journal of Clinical Medicine, 2021 Aug; 10(15): 3305
“Activation-induced marker assays identified specific T-helper cells and central memory T-cells in 80% of participants at a 12-month follow-up. In correlative analyses, older age and a longer duration of the acute phase of COVID-19 were associated with higher humoral and T-cell responses. A weak correlation between long-term loss of taste/smell and low IgA levels was found at early time points. These data indicate a long-lasting immunological memory against SARS-CoV-2 after mild COVID-19”. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8347559/
73) Dopico et al.: Immunity to SARS‐CoV‐2 induced by infection or vaccination. Journal of Internal Medicine, 2022 Jan; 291(1): 32–50
“Evidence suggests that Ab immunity to endemic seasonal CoVs and SARS‐CoV wanes within a 2‐ to 3‐year period in the majority of those previously infected… Several case studies of SARS‐CoV‐2 reinfection have been documented in the literature…., although it is not known at present whether these were caused by infection with a different strain, waning immunity or unique clinical features explained the occurrence”. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8447342/
74) Petersen et al.: SARS-CoV-2 Natural Antibody Response Persists for at Least 12 Months in a Nationwide Study From the Faroe Islands. Open Forum Infectious Diseases, 2021 Aug; 8(8): ofab378
”Although the protective role of antibodies is currently unknown, our results show that SARS-CoV-2 antibodies persisted at least 12 months after symptom onset and maybe even longer, indicating that COVID-19-convalescent individuals may be protected from reinfection. Our results represent SARS-CoV-2 antibody immunity in nationwide cohorts in a setting with few undetected cases [7], and we believe that our results add to the understanding of natural immunity”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8360234/
75) Sariol et al.: Function is more reliable than quantity to follow up the humoral response to the Receptor Binding Domain of SARS- CoV-2 Spike protein after natural infection or COVID-19 vaccination. Viruses, 2021 Oct; 13(10): 1972
“No differences were observed between naturally infected and vaccinated individuals when total anti-S antibodies and IgG titers were measured…This work is an important contribution to understanding the natural immune response to the novel coronavirus in a population severely impacted by SARS-CoV-2. Furthermore, by comparing the dynamics of the immune response after the natural infection vs. the vaccination, these findings suggest that a functional neutralizing antibody tests are more relevant indicators than the presence or absence of binding antibodies…
Furthermore, our data indicates that—compared with mRNA vaccination—natural infection induces a more robust humoral immune response in unexposed subjects.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8183028/ (pre-print-version)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8538099/
76) Molodtsov et al.: SARS-CoV-2 specific T cells and antibodies in COVID-19 protection: a prospective study. Clinical infectious Diseases, 2022 Jul 1; 75(1): e1–e9.
“Explore the impact of T cells and to quantify the protective levels of the immune responses…5,340 Moscow residents were evaluated for the antibody and cellular immune responses to SARS-CoV-2 and monitored for COVID-19 up to 300 days. The antibody and cellular responses were tightly interconnected, their magnitude inversely correlated with infection probability. Similar maximal level of protection was reached by individuals positive for both types of responses and by individuals with antibodies alone…T cells in the absence of antibodies provided an intermediate level of protection.”
https://www.medrxiv.org/content/10.1101/2021.08.19.21262278v2
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9047235/
77) A. Redd et al.: CD8+ T-Cell Responses in COVID-19 Convalescent Individuals Target Conserved Epitopes From Multiple Prominent SARS-CoV-2 Circulating Variants. Open Forum Infectious Diseases, 2021 Jul; 8(7): ofab143.
“This study examined whether CD8+ T-cell responses from coronavirus disease 2019 convalescent individuals (n = 30) potentially maintain recognition of the major severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants (alpha, beta, gamma; n = 45 mutations assessed). Only 1 mutation found in Beta variant-spike overlapped with a previously identified epitope (1/52), suggesting that virtually all anti-SARS-CoV-2 CD8+ T-cell responses should recognize these newly described variants”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8083629/
78) Mishra et al.: Natural immunity against COVID-19 significantly reduces the risk of reinfection: findings from a cohort of sero-survey participant. MedRxiv,
https://doi.org/10.1101/2021.07.19.21260302 (pre-print)
”Out of the 2238 participants, 1170 were sero-positive and 1068 were sero-negative for antibody against COVID-19. Our survey found that only 3 individuals in the sero-positive group got infected with COVID-19 whereas 127 individuals reported contracting the infection the sero-negative group. Interestingly, from the 127 sero-negative individuals who later contracted COVID-19 infection, 30 needed hospitalization, out of which 12 were on oxygen therapy, four in ICU and one was on ventilator. At the other hand, from the 3 sero-positives re-infected with COVID-19, one had hospitalization, but did not require oxygen support or critical care. These findings reinforce the strong plausibility that development of antibody following natural infection not only protects against re-infection by the virus to a great extent, but also safeguards against progression to severe COVID-19 disease”.
https://www.medrxiv.org/content/10.1101/2021.07.19.21260302v1
79) Letizia et al.: SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: a prospective cohort study. Lancet Respiratory Medicine, 2021 Jul; 9(7): 712–720.
”Seropositive young adults had about one-fifth the risk of subsequent infection compared with seronegative individuals. Although antibodies induced by initial infection are largely protective, they do not guarantee effective SARS-CoV-2 neutralisation activity or immunity against subsequent infection”.
https://pubmed.ncbi.nlm.nih.gov/33865504/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8049591/
80) Hoehl et al.: A new group at increased risk of a SARS-CoV-2 infection emerges: The recently vaccinated. Vaccine, 2021 Jul 5; 39(30): 4025–4026.
“..in the days following the start of the vaccination campaign, a new development became evident: Several of the recently vaccinated HCWs developed symptoms of Covid-19, and an infection with SARS-CoV-2 was confirmed by PCR… The recently vaccinated made up 35% of the total number of the 20 employees of the University Hospital who tested positive in that time period, meaning the recently vaccinated were overrepresented among those with a symptomatic infection”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8185246/
81) D. Rosenberg: Natural infection vs vaccination: Which gives more protection? Nearly 40% of new COVID patients were vaccinated – compared to just 1% who had been infected previously. Israel National News, Article, Jul 13, 2021, 9:24 AM (GMT+3)
“With a total of 835,792 Israelis known to have recovered from the virus, the 72 instances of reinfection amount to 0.0086% of people who were already infected with COVID…By contrast, Israelis who were vaccinated were 6.72 times more likely to get infected after the shot than after natural infection, with over 3,000 of the 5,193,499, or 0.0578%, of Israelis who were vaccinated getting infected in the latest wave.”
https://www.israelnationalnews.com/news/309762
82) D. Kwon: This ‘super antibody’ for COVID fights off multiple coronaviruses. Nature, 2021 Jul 14. doi: 10.1038/d41586-021-01917-9
“Scientists have uncovered an antibody that can fight off not only a wide range of SARS-CoV-2 variants, but also closely related coronaviruses… The researchers examined 12 antibodies that Vir Biotechnology, a company based in San Francisco, California, that was involved in the study, isolated from people who had been infected with either SARS-CoV-2 or its close relative SARS-CoV… The other 11 antibodies could target a variety of viruses, but the more effectively an antibody blocked the entry of the earliest known SARS-CoV-2 strain into a cell, the smaller the range of viruses it could bind. The team also found that antibodies that could disable a wide variety of viruses targeted sections of the binding domain that tended not to change as the virus evolved… It’s good news that the team has identified antibodies that can bind to a range of sarbecoviruses”.
https://www.nature.com/articles/d41586-021-01917-9
https://pubmed.ncbi.nlm.nih.gov/34262194/
83) Bertolini et al.: Associations of Vaccination and of Prior Infection With Positive PCR Test Results for SARS-CoV-2 in Airline Passengers Arriving in Qatar. JAMA, 2021 Jul 13; 326(2): 185–188
“Vaccination and prior infection were associated with reduced risk for SARS-CoV-2 PCR test positivity in residents of Qatar returning on international flights”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8190701/
84) Fraley et al.: Cross-reactive antibody immunity against SARS-CoV-2 in children and adults. Celluar and Molecular immunity, 2021 Jul; 18(7): 1826–1828.
”We determined that children and adults without SARS-CoV-2 infection history had preexisting cross-reactive humoral immunity that may contribute to disease pathogenesis. However, cross-reactive humoral immunity to SARS-CoV-2 is only one aspect of the immune system that may play a protective role in preventing severe COVID-19. Indeed, it has also been demonstrated that high frequencies of uninfected individuals mount preexisting cross-reactive T cell immune responses to SARS-CoV-2.. The identification of these broad B and T cell epitopes with protective efficacy might serve as future targets for pancoronavirus vaccines and also predict disease severity after coronavirus infection”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8165340/
85) N. Kojima et al.: Incidence of Severe Acute Respiratory Syndrome Coronavirus-2 infection among previously infected or vaccinated employees. MedRxiv, doi: https://doi.org/10.1101/2021.07.03.21259976 (pre-print)
”Previous SARS-CoV-2 infection and vaccination for SARS-CoV-2 were associated with decreased risk for infection or re-infection with SARS-CoV-2 in a routinely screened workforce. The was no difference in the infection incidence between vaccinated individuals and individuals with previous infection”.
https://www.medrxiv.org/content/10.1101/2021.07.03.21259976v2
86) Greany et al.: Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Science Translational Medicine, 2021 Jun 30; 13(600): eabi9915.
”The neutralizing activity of vaccine-elicited antibodies was more targeted to the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein compared to antibodies elicited by natural infection. However, within the RBD, binding of vaccine-elicited antibodies was more broadly distributed across epitopes compared to infection-elicited antibodies. This greater binding breadth means that single RBD mutations have less impact on neutralization by vaccine sera compared to convalescent sera. Therefore, antibody immunity acquired by natural infection or different modes of vaccination may have a differing susceptibility to erosion by SARS-CoV-2 evolution… recent study suggests that mRNA vaccination elicits a different distribution of isotypes and fewer antibodies that cross-react to common-cold coronaviruses as compared to infection”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8369496/
87) Karl Friston: Up to 80% not even susceptible to Covid-19. Unherd, F. Sayers, June 4, 2020
“Results have just been published of a study suggesting that 40%-60% of people who have not been exposed to coronavirus have resistance at the T-cell level from other similar coronaviruses like the common cold…the true portion of people who are not even susceptible to Covid-19 may be as high as 80%.”
https://unherd.com/2020/06/karl-friston-up-to-80-not-even-susceptible-to-covid-19/
88) Lafon et al.:
Potent SARS-CoV-2-Specific T Cell Immunity and Low Anaphylatoxin Levels Correlate With Mild Disease Progression in COVID-19 Patients. Frontiers in Immunology, 2021; 12: 684014.
“Our data clearly demonstrates a significantly stronger induction of SARS-CoV-2 specific CD8+ T lymphocytes and higher IFNγ production in patients with mild compared to patients with severe or critical COVID-19. In all patients SARS-CoV-2-specific antibodies with similar neutralizing activity were detected, but highest titers of total IgGs were observed in critical patients. Finally, elevated anaphylatoxin C3a and C5a levels were identified in severe and critical COVID-19 patients probably caused by aberrant immune complex formation due to elevated antibody titers in these patients. Crucially, we provide a full picture of cellular and humoral immune responses of COVID-19 patients and prove that robust polyfunctional CD8+ T cell responses concomitant with low anaphylatoxin levels correlate with mild infections. In addition, our data indicates that high SARS-CoV-2 antibody titers are associated with severe disease progression”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8237940/
89) Nelde et al.: SARS-CoV-2 T-cell epitopes define heterologous and COVID-19-induced T-cell recognition. Nature Immunology, 2020 Sept., 22, pages74–85 (2021)
“The first work identifying and characterizing SARS-CoV-2-specific and cross-reactive HLA class I and HLA-DR T-cell epitopes in SARS-CoV-2 convalescents (n = 180) as well as unexposed individuals (n = 185) and confirming their relevance for immunity and COVID-19 disease course…cross-reactive SARS-CoV-2 T-cell epitopes revealed pre-existing T-cell responses in 81% of unexposed individuals, and validation of similarity to common cold human coronaviruses provided a functional basis for postulated heterologous immunity in SARS-CoV-2 infection…intensity of T-cell responses and recognition rate of T-cell epitopes was significantly higher in the convalescent donors compared to unexposed individuals, suggesting that not only expansion, but also diversity spread of SARS-CoV-2 T-cell responses occur upon active infection.”
https://www.researchsquare.com/article/rs-35331/v1%20%20pubmed
https://www.nature.com/articles/s41590-020-00808-x
90) Ciocca et al.: Evolution of Human Memory B Cells From Childhood to Old Age. Frontiers in Immunology, 2021; 12: 690534.
“After in vitro stimulation with CpG, B cells from older individuals produced significantly fewer IgM and IgA antibodies compared to younger individuals. Aging is a complex process characterized by a functional decline in multiple physiological systems. The immune system of older people is well equipped to react to often encountered antigens but has a low ability to respond to new pathogens”. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8343175/
91) R. Sealy et al.:
Cross-Reactive Immune Responses toward the Common Cold Human Coronaviruses and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Mini-Review and a Murine Study. Microorganisms, 2021 Jul 31;9(8):1643.
”Når mennesker blev naturligt inficeret med SARS-CoV-2, steg krydsreaktive antistoffer, der genkendte almindelig forkølelses-HCoV-antigener, i betydeligt omfang. Krydsreaktive T-celler, ligesom antistoffer, var til stede i mennesker før SARS-CoV-2-eksponeringer og steg efter SARS-CoV-2-infektioner. Nogle undersøgelser antydede, at humane infektioner med almindelige forkølelses-HCoV’er gav beskyttelse mod sygdom forårsaget af efterfølgende eksponeringer for SARS-CoV-2”.
https://pubmed.ncbi.nlm.nih.gov/34442723/
92) H. Abo-Leyah et al.: The protective effect of SARS-CoV-2 antibodies in Scottish healthcare workers. ERJ Open Research,2021 Apr; 7(2): 00080-2021
“HCWs were three times more likely to test positive for SARS-CoV-2 than the general population. Almost all infected individuals developed an antibody response, which was 85% effective in protecting against re-infection with SARS-CoV-2”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8164012/
93) Björkström et al.:
Natural killer cells in antiviral immunity. Nature Reviews Immunology, 2022; 22(2): 112–123.
“Importantly, NK cells can also eliminate virus-infected cells via CD16-mediated antibody-dependent cellular cytotoxicity (ADCC)16. Finally, NK cell activity is modulated by cytokines, including, but not limited to, the activating cytokines IL-2, IL-12, IL-15, IL-18 and type I interferons, which can be produced by virally infected cells or activated antigen-presenting cells17. Many of these cytokines, alone or in combination, promote NK cell survival, proliferation, cytotoxicity and cytokine production, including interferon-γ (IFNγ) production18. Through these mechanisms, NK cells are uniquely equipped to sense and quickly respond to viral infections7. In this respect, they often act in concert with other host immune responses in mediating antiviral immunity”.
https://www.nature.com/articles/s41577-021-00558-3
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8194386/
94) Cohen et al.: Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Reports Medicine, 2021 Jul 20; 2(7): 100354.
“Here, we evaluate 254 COVID-19 patients longitudinally up to 8 months and find durable broad-based immune responses. SARS-CoV-2 spike binding and neutralizing antibodies exhibit a bi-phasic decay with an extended half-life of >200 days suggesting the generation of longer-lived plasma cells. SARS-CoV-2 infection also boosts antibody titers to SARS-CoV-1 and common betacoronaviruses. In addition, spike-specific IgG+ memory B cells persist, which bodes well for a rapid antibody response upon virus re-exposure or vaccination. Virus-specific CD4+ and CD8+ T cells are polyfunctional and maintained with an estimated half-life of 200 days. Interestingly, CD4+ T cell responses equally target several SARS-CoV-2 proteins, whereas the CD8+ T cell responses preferentially target the nucleoprotein, highlighting the potential importance of including the nucleoprotein in future vaccines. Taken together, these results suggest that broad and effective immunity may persist long-term in recovered COVID-19 patients”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8253687/
The study is based on the studyLongitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8095229/ (preprint)
95) Jung et al.: SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nature Communications, 2021; 12: 4043.
”In the present study, we report SARS-CoV-2-specific CD4+ and CD8+ T cell responses in peripheral blood mononuclear cells (PBMCs) from individuals infected with SARS-CoV-2 over 10 months post-infection. Using diverse T cell assays, we show that SARS-CoV-2-specific memory T cell responses are maintained 10 months after the infection. In addition, we report the differentiation of SARS-CoV-2-specific memory T cells during the study period and the successful generation of TSCM cells.
We demonstrated that PD-1 and TIGIT are rarely expressed in SARS-CoV-2-specific TSCM cells, indicating that SARS-CoV-2-specific TSCM cells are not exhausted-like progenitors, but bona fide stem-like memory cells.
We also demonstrate the effector functions and the proliferation capacity of long-term memory CD4+ and CD8+ T cells. Our findings provide insights for understanding long-term SARS-CoV-2-specific T cell immunity”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8245549/
96) Shrestha et al.:
Necessity of COVID-19 Vaccination in Persons Who Have Already Had COVID-19. Clinical Infectous Diseases, 2022 Aug 24;75(1):e662-e671
”Individuals who have had SARS-CoV-2 infection are unlikely to benefit from COVID-19 vaccination, and vaccines can be safely prioritized to those who have not been infected before.
Cumulative incidence of COVID-19 was examined among 52238 employees in an American healthcare system. COVID-19 did not occur in anyone over the five months of the study among 2579 individuals previously infected with COVID-19, including 1359 who did not take the vaccine”.
https://www.medrxiv.org/content/10.1101/2021.06.01.21258176v2
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8807217/
97) S. Nielsen et al.: SARS-CoV-2 elicits robust adaptive immune responses regardless of disease severity, Ebiomedicine. 2021 Jun; 68: 103410.
“We included 203 recovered SARS-CoV-2 infected patients in Denmark between April 3rd and July 9th 2020, at least 14 days after COVID-19 symptom recovery. The participants had experienced a range of disease severities from asymptomatic to severe…
Overall, the majority of patients had robust adaptive immune responses, regardless of their disease severity”.
https://pubmed.ncbi.nlm.nih.gov/34098342/
98) Lumley et al.: An observational cohort study on the incidence of SARS-CoV-2 infection and B.1.1.7 variant infection in healthcare workers by antibody and vaccination status. Clinical Infectious Diseases, 2022 Apr 9;74(7):1208-1219
”Compared to unvaccinated seronegative HCWs, natural immunity and 2 vaccination doses provided similar protection against symptomatic infection… There was no evidence of differences in immunity induced by natural infection and vaccination for infections with S-gene target failure and B.1.1.7”.
https://pubmed.ncbi.nlm.nih.gov/34216472/
99) S. Jordan: Innate and adaptive immune responses to SARS-CoV-2 in humans: relevance to acquired immunity and vaccine responses. Clinical and Experimental Immunology, 2021 Jun; 204(3): 310–320.
“In addition, long-term assessment of SARS-CoV-2 memory B and T cell-mediated immune responses in patients recovering from an infection or those with cross-reactive immunological memory will help to define risk for future SARS-CoV infections. Finally, patients recovering from SARS-CoV-2 infection may experience prolonged immune activation probably due to T cell exhaustion”.
https://pubmed.ncbi.nlm.nih.gov/33534923/
100) J. Turner et al.: SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature, 2021 Jul;595(7867):421-425
”Consistently, circulating resting memory B cells directed against SARS-CoV-2 S were detected in the convalescent individuals. Overall, our results indicate that mild infection with SARS-CoV-2 induces robust antigen-specific, long-lived humoral immune memory in humans”.
https://pubmed.ncbi.nlm.nih.gov/34030176/
101) L. Yao et al.: Persistence of Antibody and Cellular Immune Responses in COVID-19 patients over Nine Months after Infection. Journal of Infectious Diseases, 2021 May 12 : jiab255
”SARS-CoV-2-specific immune memory response persists in most patients nearly one year after infection, which provides a promising sign for prevention from reinfection and vaccination strategy”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8243600/
102) P. Ahluwalia et a.: Infection and Immune Memory: Variables in Robust Protection by Vaccines Against SARS-CoV-2. Frontiers Immunology, 2021; 12: 660019
“Virus-specific CD8+ T cells have been shown to persist for 6-11 years (102, 103). In another study, memory T cells against SARS-CoV-1 were detected even 17 years after the previous pandemic, hinting at the long-term immune response against the pathogens (17). In a recent study, almost half of donor blood samples between 2015 and 2018 showed reactivity to SARS-CoV-2, well before the emergence of this pathogen in the human population. It is speculated that this might be due to the cross-reactivity of other common cold coronaviruses with SARS-CoV-2”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8144450/
103) Lineburg et al.: CD8+ T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope cross-react with selective seasonal coronaviruses. Immunity, 2021 May 11; 54(5): 1055–1065.e5.
“Screening of SARS-CoV-2 peptide pools revealed that the nucleocapsid (N) protein induced an immunodominant response in HLA-B7+ COVID-19-recovered individuals that was also detectable in unexposed donors. Our findings demonstrate the basis of selective T cell cross-reactivity for an immunodominant SARS-CoV-2 epitope and its homologs from seasonal coronaviruses, suggesting long-lasting protective immunity”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8043652/
104) E. Murchu et al.: Quantifying the risk of SARS‐CoV‐2 reinfection over time. Reviews in Medical Virology, 2022 Jan; 32(1): e2260.
”Reinfection was an uncommon event (absolute rate 0%–1.1%), with no study reporting an increase in the risk of reinfection over time. Only one study estimated the population‐level risk of reinfection based on whole genome sequencing in a subset of patients; the estimated risk was low (0.1% [95% CI: 0.08–0.11%]) with no evidence of waning immunity for up to 7 months following primary infection. These data suggest that naturally acquired SARS‐CoV‐2 immunity does not wane for at least 10 months post‐infection”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8209951/
105) Ivanova et al.: Discrete Immune Response Signature to SARS-CoV-2 mRNA Vaccination Versus Infection. Sneak Peak/MedRxiv/Pubmed pre-print, 3 May 2021
“In COVID-19 patients, immune responses were characterized by a highly augmented interferon response which was largely absent in vaccine recipients. Increased interferon signaling likely contributed to the observed dramatic upregulation of cytotoxic genes in the peripheral T cells and innate-like lymphocytes in patients but not in immunized subjects.
Analysis of B and T cell receptor repertoires revealed that while the majority of clonal B and T cells in COVID-19 patients were effector cells, in vaccine recipients clonally expanded cells were primarily circulating memory cells. Importantly, the divergence in immune subsets engaged, the transcriptional differences in key immune populations, and the differences in maturation of adaptive immune cells revealed by our analysis have far-ranging implications for immunity to this novel pathogen”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8077568/
106) Bert et al.: Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. Journal of Experimental Medicine, 2021 May 3; 218(5): e20202617.
”Thus, asymptomatic SARS-CoV-2–infected individuals are not characterized by weak antiviral immunity; on the contrary, they mount a highly functional virus-specific cellular immune response”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7927662/
107) A. Breathnach et al.: Prior COVID-19 protects against reinfection, even in the absence of detectable antibodies. Journal of Infection, May 27, 2021DOI:https://doi.org/10.1016/j.jinf.2021.05.024
“Studies did not address whether prior infection is protective in the absence of a detectable humoral immune response. Patients with primary or secondary antibody deficiency syndrome and reduced or absent B cells can recover from COVID-19…Although there have been few mechanistic studies, preliminary data show that such individuals generate striking T-cell immune responses against SARS-CoV-2 peptide pools…SARS-CoV-2 specific T cell immune responses but not neutralizing antibodies are associated with reduced disease severity suggesting the immune system may have considerable redundancy or compensation following COVID-19…our results add to the emerging evidence that detectable serum antibody may be an incomplete marker of protection against reinfection. This could have implications for public health and policy-making, for example if using seroprevalence data to assess population immunity, or if serum antibody levels were to be taken as official evidence of immunity – a minority of truly immune patients have no detectable antibody and could be disadvantaged as a result. Our findings highlight the need for further studies of immune correlates of protection from infection with SARS-CoV-2, which may in turn enhance development of effective vaccines and treatments.”
https://www.journalofinfection.com/article/S0163-4453(21)00266-8/fulltext
https://pubmed.ncbi.nlm.nih.gov/34052242/
108) Abu-Raddad et al.: SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClinicalmedicine, 2021 May;35:100861. doi: 10.1016/j.eclinm.2021.100861. Epub 2021 Apr 28
”Reinfection is rare in the young and international population of Qatar. Natural infection appears to elicit strong protection against reinfection with an efficacy ~95% for at least seven months”.
https://pubmed.ncbi.nlm.nih.gov/33937733/
109) P. Ahluwalia et al.: Infection and Immune Memory: Variables in Robust Protection by Vaccines Against SARS-CoV-2. Frontiers in Immunology, 2021; 12: 660019.
“Although neutralizing antibodies can wane over time, long-lasting B and T memory cells can persist in recovered individuals. The natural immunological memory captures the diverse repertoire of SARS-CoV-2 epitopes after natural infection whereas, currently approved vaccines are based on a single epitope, spike protein”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8144450/
110) J. Vitale et al.: Assessment of SARS-CoV-2 Reinfection 1 Year After Primary Infection in a Population in Lombardy, Italy. JAMA Internal Medicine, 2021 Oct; 181(10): 1407–1408.
”The study results suggest that reinfections are rare events and patients who have recovered from COVID-19 have a lower risk of reinfection. Natural immunity to SARS-CoV-2 appears to confer a protective effect for at least a year, which is similar to the protection reported in recent vaccine studies”.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2780557
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8164145/
111) Föhse et al.: The BNT162b2 mRNA vaccine against SARS-CoV-2 reprograms both adaptive and innate immune response. MedRxiv, May 06, 2021, doi: https://doi.org/10.1101/2021.05.03.21256520 (Pre-print)
“ The response of innate immune cells to TLR4 and TLR7/8 ligands was lower after BNT162b2 vaccination, while fungi-induced cytokine responses were stronger. In conclusion, the mRNA BNT162b2 vaccine induces complex functional reprogramming of innate immune responses, which should be considered in the development and use of this new class of vaccines”.
https://www.medrxiv.org/content/10.1101/2021.05.03.21256520v1
112) WHO: COVID-19 natural immunity, Scientific brief. World Health Organization, 10 May 2021
”Available scientific data suggests that in most people immune responses remain robust and protective against reinfection for at least 6-8 months after infection (the longest follow up with strong scientific evidence is currently approximately 8 months). Some variant SARS-CoV-2 viruses with key changes in the spike protein have a reduced susceptibility to neutralization by antibodies in the blood. While neutralizing antibodies mainly target the spike protein, cellular immunity elicited by natural infection also target other viral proteins, which tend to be more conserved across variants than the spike protein”.
Current evidence points to most individuals developing strong protective immune responses following natural infection with SARSCoV-2. However, inaccurate immunodiagnostic tests may falsely indicate infected individuals as naïve to the virus (not previously infected) or may falsely label non-infected people as positive for immune markers of recent infection”.
https://apps.who.int/iris/bitstream/handle/10665/341241/WHO-2019-nCoV-Sci-Brief-Natural-immunity-2021.1-eng.pdf?sequence=3&isAllowed=y
113) E. Callaway: Had COVID? You’ll probably make antibodies for a lifetime. Nature, 2021 May 26. doi: 10.1038/d41586-021-01442-9.
“People who recover from mild COVID-19 have bone-marrow cells that can churn out antibodies for decades…the study provides evidence that immunity triggered by SARS-CoV-2 infection will be extraordinarily long-lasting.”
https://www.nature.com/articles/d41586-021-01442-9
https://pubmed.ncbi.nlm.nih.gov/34040250/
114) Goldberg et al.: Protection of previous SARS-CoV-2 infection is similar to that of BNT162b2 vaccine protection: A three-month nationwide experience from Israel. American Journal of Epidemiology, 2022 Mar 30: kwac060
“Our results should be considered by policymakers when deciding whether or not to prioritize vaccination of previously-infected adults”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8992290/
115) Alfego et al.: A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States. EClinicalmedicine, 2021 Jun;36:100902
“To track population-based SARS-CoV-2 antibody seropositivity duration across the United States using observational data from a national clinical laboratory registry of patients tested by nucleic acid amplification (NAAT) and serologic assays… specimens from 39,086 individuals with confirmed positive COVID-19…both S and N SARS-CoV-2 antibody results offer an encouraging view of how long humans may have protective antibodies against COVID-19, with curve smoothing showing population seropositivity reaching 90% within three weeks, regardless of whether the assay detects N or S-antibodies. Observational data from a national clinical laboratory, though limited by an epidemiological view of the U.S. population, offer an encouraging timeline for the development and sustainability of antibodies up to ten months from natural infection and could inform post-pandemic planning”.
https://www.thelancet.com/action/showPdf?pii=S2589-5370(21)00182-6
https://pubmed.ncbi.nlm.nih.gov/34056568/
116) Majdoubi et al.: A majority of uninfected adults show preexisting antibody reactivity against SARS-CoV-2. JCI Insight. 2021 Apr 22; 6(8): e146316.
“Preexisting cross-reactivity to SARS-CoV-2 occurs in the absence of prior viral exposure…. we determined that more than 90% of uninfected adults showed antibody reactivity against the spike protein, receptor-binding domain (RBD), N-terminal domain (NTD), or the nucleocapsid (N) protein from SARS-CoV-2…
The common occurrence of circulating coronaviruses year after year and their structural similarity with SARS-CoV-2 raises the possibility that the former may stimulate cross-reactive responses toward SARS-CoV-2 and that this heterotopic immunity may impact clinical susceptibility to COVID-19…
We conclude that most adults display preexisting antibody cross-reactivity against SARS-CoV-2, which further supports investigation of how this may impact the clinical severity of COVID-19”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8119195/
117) C. Ogega et al.: Durable SARS-CoV-2 B cell immunity after mild or severe disease. The Journal of Clinical Investigation, 2021 Apr 1; 131(7): e145516.
“Multiple studies have shown loss of severe acute respiratory syndrome coronavirus 2–specific (SARS-CoV-2–specific) antibodies over time after infection, raising concern that humoral immunity against the virus is not durable. If immunity wanes quickly, millions of people may be at risk for reinfection after recovery from coronavirus disease 2019 (COVID-19). However, memory B cells (MBCs) could provide durable humoral immunity even if serum neutralizing antibody titers decline…. Resting MBCs (rMBCs) made up the largest proportion of S-RBD–specific MBCs in both cohorts. FCRL5, a marker of functional memory on rMBCs, was more dramatically upregulated on S-RBD–specific rMBCs after mild infection than after severe infection. These data indicate that most SARS-CoV-2-infected individuals develop S-RBD–specific, class-switched rMBCs that resemble germinal center–derived B cells induced by effective vaccination against other pathogens, providing evidence for durable B cell–mediated immunity against SARS-CoV-2 after mild or severe disease”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8011891/
118) V. Hall et al.: SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). The Lancet, 021 17-23 April; 397(10283): 1459–1469.
“A previous history of SARS-CoV-2 infection was associated with an 84% lower risk of infection, with median protective effect observed 7 months following primary infection. This time period is the minimum probable effect because seroconversions were not included. This study shows that previous infection with SARS-CoV-2 induces effective immunity to future infections in most individuals”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8040523/
119) G. Anichini et al.: SARS-CoV-2 Antibody Response in Persons with Past Natural Infection. New England Journal of Medicine, 2021 Apr 14 : NEJMc2103825.
”The most remarkable finding of this study was the significantly lower neutralizing antibody titer after administration of a second dose of vaccine in previously uninfected patients than the titer after only a single dose of vaccine in previously infected participants. It is unclear how the neutralizing antibody titers influence the ability of the host to transmit the virus. These findings provide evidence that after the administration of a single dose of vaccine, the humoral response against SARS-CoV-2 in persons with a history of SARS-CoV-2 infection is greater than the response in previously uninfected participants who have received a second dose”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8063888/
120) Saini et al.: SARS-CoV-2 genome-wide T cell epitope mapping reveals immunodominance and substantial CD8+ T cell activation in COVID-19 patients. Science Immunology, 2021 Apr 14; 6(58): eabf7550
“Most vaccine development efforts are currently focusing on mounting antibody responses to the spike protein, with limited focus on T cell immunity…
Taken together, COVID-19 disease drives substantial T cell activation, with T cell recognition of a large number of SARS-CoV-2-derived peptides. There is also considerable T cell recognition of such peptides in healthy donors, arguing for cross-recognition, potentially from T cells raised against other coronaviruses… Data suggest a lower TCR avidity to the probed pMHC in healthy individuals compared to COVID-19 patients, which could represent potential cross-reactivity from existing T cell populations potentially raised against other coronaviruses (such as common cold viruses HCoV-HKU1, HCoV-229E, HCoV-NL63, and HCoV-OC43) that share some level of sequence homology with SARS-CoV-2, as suggested in recent reports… Importantly the phenotype of the SARS-CoV-2-specific CD8+ T cells, revealed a strong T cell activation in COVID-19 patients, while minimal T cell activation was seen in healthy individuals. We found that patients with severe disease displayed significantly larger SARS-CoV-2-specific T cell populations compared to patients with mild diseases and these T cells displayed a robust activation profile. These results further our understanding of T cell immunity to SARS-CoV-2 infection and hypothesize that strong antigen-specific T cell responses are associated with different disease outcomes”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8139428/
121) B. Laidlaw et al.: Transcriptional regulation of memory B cell differentiation. Nature Rewies Immunology, 2021 Apr;21(4):209-220
””Memory B cells (MBCs) are critical for the rapid development of protective immunity following re-infection. MBCs capable of neutralizing distinct subclasses of pathogens, such as influenza and HIV, have been identified in humans. However, efforts to develop vaccines that induce broadly protective MBCs to rapidly mutating pathogens have not yet been successful”.
https://pubmed.ncbi.nlm.nih.gov/33024284/
122) Cromer et al.: Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nature Rewievs Immunology, 2021 Jun;21(6):395-404
“The waning of neutralizing antibody responses over the first year after infection suggests that reinfections may become more frequent in the coming months and/or years. However, the robust B cell and T cell memory responses induced by primary infection suggest that reinfection severity, and potentially transmission, may be mitigated over the longer term”.
https://pubmed.ncbi.nlm.nih.gov/33927374/
123) L. Atlani-Duault et al.: Immune evasion means we need a new COVID-19 social contract. The Lancet Public Health, 2021 Apr; 6(4): e199–e200.
”This virological game changer has numerous consequences, not only for vaccines and treatment, but also for prevention and control strategies. The fervently awaited end of this global health crisis might be continually postponed, as new variants emerge and immune evasion reduces vaccination effectiveness in the short and medium term.
Hence, it is time to abandon fear-based approaches based on seemingly haphazard stop-start generalised confinement as the main response to the pandemic; approaches which expect citizens to wait patiently until intensive care units are re-enforced, full vaccination is achieved, and herd immunity is reached…
We scientists working against COVID-19 must have the courage to address those in power, who bear ultimate responsibility for the policies chosen and their consequences. If this responsibility is shirked or delayed, the inevitable day of reckoning might be terrible.
We are members of the French COVID-19 Scientific Council”.
https://www.thelancet.com/journals/lanpub/article/PIIS2468-2667(21)00036-0/fulltext
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7891047/
124) R. Goel et al.: Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Science Immunology, 2021 Apr 15; 6(58): eabi6950.
“In SARS-CoV-2 recovered individuals, antibody and memory B cell responses were significantly boosted after the first vaccine dose; however, there was no increase in circulating antibodies, neutralizing titers, or antigen-specific memory B cells after the second dose. This robust boosting after the first vaccine dose strongly correlated with levels of pre-existing memory B cells in recovered individuals, identifying a key role for memory B cells in mounting recall responses to SARS-CoV-2 antigens”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8158969/
125) J. Wu et al.: SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nature Communications,
2021; 12: 1813.
“…data indicate sustained humoral immunity in recovered patients who had symptomatic COVID-19, suggesting prolonged immunity”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7985370/
126) Perez et al.: A 1 to 1000 SARS-CoV-2 reinfection proportion in members of a large healthcare provider in Israel: a preliminary report. MedRxiv, doi: https://doi.org/10.1101/2021.03.06.21253051 (pre-print)
Israel: “Out of 149,735 individuals with a documented positive PCR test between March 2020 and January 2021, 154 had two positive PCR tests at least 100 days apart, reflecting a reinfection proportion of 1 per 1000.”
https://www.medrxiv.org/content/10.1101/2021.03.06.21253051v1
127) Sheehan et al.: Reinfection Rates among Patients who Previously Tested Positive for COVID-19: a Retrospective Cohort Study. Clinical Infectious Diseases, 2021 Mar 15: ciab234.
“This retrospective cohort study of one multi-hospital health system included 150,325 patients tested for COVID-19 infection via PCR from March 12, 2020 to August 30, 2020.
Prior infection in patients with COVID-19 was highly protective against reinfection and symptomatic disease. This protection increased over time, suggesting that viral shedding or ongoing immune response may persist beyond 90 days and may not represent true reinfection”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7989568/
128) J. Klausner et al.: Op-Ed: Quit Ignoring Natural COVID Immunity – Antibody testing and proof of prior infection can allow more people to return to normal. Medpagetoday, May 28, 2021
”So why are we so focused on vaccine-induced immunity – in our goals to reach herd immunity, our gatekeeping on travel, public or private events, or mask use – while ignoring natural immunity? Shouldn’t those who have natural immunity also be able to return to “normal” activities”? https://www.medpagetoday.com/infectiousdisease/covid19/92836?xid=nl_secondopinion_2021-06-01&eun=g1666187d0r
129) Ansari et al.: Immune Memory in Mild COVID-19 Patients and Unexposed Donors Reveals Persistent T Cell Responses After SARS-CoV-2 Infection. Frontiers in immunology, 2021; 12: 636768
”We found detectable immune memory in mild COVID-19 patients several months after recovery in the crucial arms of protective adaptive immunity; CD4+ T cells and B cells, with a minimal contribution from CD8+ T cells. Interestingly, the persistent immune memory in COVID-19 patients is predominantly targeted towards the Spike glycoprotein of the SARS-CoV-2. This study provides the evidence of both high magnitude pre-existing and persistent immune memory in Indian population”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7991090/
130) Sokal et al.: Maturation and persistence of the anti-SARS-CoV-2 memory B cell response. Cell, 2021 Mar 4; 184(5): 1201–1213.e14
“Memory B cells play a fundamental role in host defenses against viruses, but to date, their role has been relatively unsettled in the context of SARS-CoV-2. We report here a longitudinal single-cell and repertoire profiling of the B cell response up to 6 months in mild and severe COVID-19 patients. Overall, these findings demonstrate that an antigen-driven activation persisted and matured up to 6 months after SARS-CoV-2 infection and may provide long-term protection”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7994111/
131) Harvey et al.: Association of SARS-CoV-2 Seropositive Antibody Test With Risk of Future Infection. JAMA Internal Medicine, 2021 May; 181(5): 672–679.
“The cohort included 3 257 478 unique patients. In this cohort study, patients with positive antibody test results were initially more likely to have positive NAAT results, consistent with prolonged RNA shedding, but became markedly less likely to have positive NAAT results over time, suggesting that seropositivity is associated with protection from infection. The duration of protection is unknown, and protection may wane over time”. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7905701/
132) J. Zuo et al.: Robust SARS-CoV-2-specific T-cell immunity is maintained at 6 months following primary infection. Nature Immunology, 2021 May 1; 22(5): 620–626.
“Median T-cell responses were 50% higher in donors who had experienced a symptomatic infection indicating that the severity of primary infection establishes a ‘setpoint’ for cellular immunity. T-cell responses to spike and nucleoprotein/membrane proteins were correlated with peak antibody levels. In conclusion, our data are reassuring that functional SARS-CoV-2-specific T-cell responses are retained at six months following infection”. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7610739/
133) Tong et al.: Memory B cell repertoire for recognition of evolving SARS-CoV-2 spike. Cell, 2021 Sep 16; 184(19): 4969–4980.e15.
“Memory B cell reserves can generate protective antibodies against repeated SARS-CoV-2 infections, but with unknown reach from original infection to antigenically drifted variants. We charted memory B cell receptor-encoded antibodies from 19 COVID-19 convalescent subjects against SARS-CoV-2 spike (S) and found seven major antibody competition groups against epitopes recurrently targeted across individuals… Inclusion of published and newly determined structures of antibody-S complexes identified corresponding epitopic regions.. Group assignment correlated with cross-CoV-reactivity breadth, neutralization potency, and convergent antibody signatures…
Although emerging SARS-CoV-2 variants of concern escaped binding by many members of the groups associated with the most potent neutralizing activity, some antibodies in each of those groups retained affinity—suggesting that otherwise redundant components of a primary immune response are important for durable protection from evolving pathogens.
Our results furnish a global atlas of S-specific memory B cell repertoires and illustrate properties driving viral escape and conferring robustness against emerging variants”.
https://www.biorxiv.org/content/10.1101/2021.03.10.434840v1.full
(”Our results provide a global atlas of S-specific memory B cell repertoires and illustrate properties that drive virus escape and confer robustness against new variants”) https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8299219/
134) K. Mølbak et al.: Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. The Lancet, 2021 27 March-2 April; 397(10280): 1204–1212.
“Protection against repeat infection was 80·5%. We found no difference in estimated protection against repeat infection by sex (male 78·4% [72·1–83·2] vs female 79·1% [73·9–83·3]) or evidence of waning protection over time (3–6 months of follow-up 79·3% [74·4–83·3] vs ≥7 months of follow-up 77·7% [70·9–82·9]).
Interpretation: Our findings could inform decisions on which groups should be vaccinated and advocate for vaccination of previously infected individuals because natural protection, especially among older people, cannot be relied on”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7969130/
135) Lozano-Ojalvo et al.: Differential effects of the second SARS-CoV-2 mRNA vaccine dose on T cell immunity in naive and COVID-19 recovered individuals. Cell Reports, 2021 Aug 24; 36(8): 109570.
”Overall, our data support the vaccination scheme determined in clinical trials with prompt administration of the second dose in individuals without previous SARS-CoV-2 exposure (Kadire et al., 2021), but they suggest that in individuals with pre-existing immunity against SARS-CoV-2 the second dose of the vaccine may not be necessary.
Our results demonstrate that, while the second dose increases both the humoral and cellular immunity in naive individuals, COVID-19 recovered individuals reach their peak of immunity after the first dose. These results suggest that a second dose, according to the current standard regimen of vaccination, may be not necessary in individuals previously infected with SARS-CoV-2”.
“In naive subjects, the T cell response is boosted in conjunction with spike-specific IgG levels after the second dose. On the contrary, the second vaccine dose not increase the spike-specific T cell response in individuals with a pre-existing immunity against SARS-CoV-2 and further evidences that a single-dose vaccine is sufficient to induce protective immunity in Covid-19 recovered individuals
.” https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8332924/
136) H. Kared et al.: SARS-CoV-2–specific CD8+ T cell responses in convalescent COVID-19 individuals. Journal of Clinical Investigation, 2021 Mar 1; 131(5): e145476.
“A multiplexed peptide-MHC tetramer approach was used to screen 408 SARS-CoV-2 candidate epitopes for CD8+ T cell recognition in a cross-sectional sample of 30 coronavirus disease 2019 convalescent individuals…Modelling demonstrated a coordinated and dynamic immune response characterized by a decrease in inflammation, increase in neutralizing antibody titer, and differentiation of a specific CD8+ T cell response. Overall, T cells exhibited distinct differentiation into stem cell and transitional memory states (subsets), which may be key to developing durable protection.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7919723/
137) Brolin et al.: Risk of SARS-CoV-2 transmission from newly infected individuals with documented previous infection or vaccination. European Centre for Disease Prevention and Control, 2021 March 29.
“The review of evidence on natural immunity and possibilities for transmission from previously infected to susceptible contacts found that
– There is evidence that reinfection remains a rare event…
Results from cohort studies confirm that the protective effect of previous SARS-CoV-2 infection ranges from 81% to 100% from Day 14 following initial infection, for a follow-up period of five to seven months. Protection against reinfection is lower in individuals aged 65 years and older…
These studies were carried out before the emergence of SARS-CoV-2 variants of concern (VOCs) and therefore there is limited preliminary evidence that immunity induced against previously circulating SARS-CoV-2 variants may not have the same potency or duration against the VOCs identified to date (in particular the B.1.351 and P.1 variants.) • As the number of individuals acquiring natural immunity increases, the total number of infections is expected to decrease significantly, leading to decreased transmission overall, unless the genetic changes in the circulating variants induce significant immune escape”.
https://www.ecdc.europa.eu/sites/default/files/documents/Risk-of-transmission-and-reinfection-of-SARS-CoV-2-following-vaccination.pdf
138) Pilz et al.: SARS‐CoV‐2 re‐infection risk in Austria. European Journal of Clinical investigation,
2021 Apr; 51(4): e13520
“We observed a relatively low re-infection rate of SARS-CoV-2 in Austria. Protection against SARS-CoV-2 after natural infection is comparable with the highest available estimates on vaccine efficacies. Further well-designed research on this issue is urgently needed for improving evidence-based decisions on public health measures and vaccination strategies”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7988582/
139) Bertoletti et al.: The T cell response to SARS-CoV-2: kinetic and quantitative aspects and the case for their protective role. Oxford Open immunology,
2021 Feb 23: iqab006
“SARS-CoV-specific T cells (CD4 and CD8 T cells) can be detected at 11 and 17 years after SARS-CoV infection [17, 76] in the absence of detectable antibodies… Early appearance, multi-specificity and functionality of SARS-CoV-2-specific T cells are associated with accelerated viral clearance and with protection from severe COVID-19”.
https://academic.oup.com/ooim/article/2/1/iqab006/6146940?login=false
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7928654/
140) Sette et al.: Adaptive immunity to SARS-CoV-2 and COVID-19. Cell, 2021 Feb 18; 184(4): 861–880.
“The adaptive immune system is important for control of most viral infections. The three fundamental components of the adaptive immune system are B cells (the source of antibodies), CD4+ T cells, and CD8+ T cells…a picture has begun to emerge that reveals that CD4+ T cells, CD8+ T cells, and neutralizing antibodies all contribute to control of SARS-CoV-2 in both non-hospitalized and hospitalized cases of COVID-19.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7803150/
141) Sage et al.: Pre-existing heterosubtypic immunity provides a barrier to airborne transmission of influenza viruses. PLOS Pathogens, 2021 Feb; 17(2): e1009273.
“We found that pre-existing H3N2 immunity did not significantly block transmission of the 2009 H1N1pandemic (H1N1pdm09) virus to immune animals. Surprisingly, airborne transmission of seasonal H3N2 influenza strains was abrogated in recipient animals with H1N1pdm09 pre-existing immunity. This protection from natural infection with H3N2 virus was independent of neutralizing antibodies. Pre-existing immunity with influenza B virus did not block H3N2 virus transmission, indicating that the protection was likely driven by the adaptive immune response. We demonstrate that pre-existing immunity can impact susceptibility to heterologous influenza virus strains, and implicate a novel correlate of protection that can limit the spread of respiratory pathogens through the air”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7891786/
142) Tarke et al.: Negligible impact of SARS-CoV-2 variants on CD4+ and CD8+ T cell reactivity in COVID-19 exposed donors and vaccines. Cell Reports Medicine, 2021 Jul 20; 2(7): 100355.
“Performed a comprehensive analysis of SARS-CoV-2-specific CD4+ and CD8+ T cell responses from COVID-19 convalescent subjects recognizing the ancestral strain, compared to variant lineages B.1.1.7, B.1.351, P.1, and CAL.20C as well as recipients of the Moderna (mRNA-1273) or Pfizer/BioNTech (BNT162b2) COVID-19 vaccines…
the sequences of the vast majority of SARS-CoV-2 T cell epitopes are not affected by the mutations found in the variants analyzed. Overall, the results demonstrate that CD4+ and CD8+ T cell responses in convalescent COVID-19 subjects or COVID-19 mRNA vaccinees are not substantially affected by mutations”.
https://www.biorxiv.org/content/10.1101/2021.02.27.433180v1
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7941626/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8249675/
143) S. Reynolds: Lasting immunity found after recovery from COVID-19. National Institutes of Health, January 26, 2021
“The researchers found durable immune responses in the majority of people studied. Antibodies against the spike protein of SARS-CoV-2, which the virus uses to get inside cells, were found in 98% of participants one month after symptom onset. As seen in previous studies, the number of antibodies ranged widely between individuals. But,
promisingly, their levels remained fairly stable over time, declining only modestly at 6 to 8 months after infection… virus-specific B cells increased over time.
Several months ago, our studies showed that natural infection induced a strong response, and this study now shows that the responses last,” Weiskopf says. “We are hopeful that a similar pattern of responses lasting over time will also emerge for the vaccine-induced responses.”
https://www.nih.gov/news-events/nih-research-matters/lasting-immunity-found-after-recovery-covid-19
144) M. Shrotri et al.: T cell response to SARS-CoV-2 infection in humans: A systematic review. PLOS One, 2021; 16(1): e0245532
“A complex pattern of T cell response to SARS-CoV-2 infection has been demonstrated, but inferences regarding population level immunity are hampered by significant methodological limitations and heterogeneity between studies, as well as a striking lack of research in asymptomatic or pauci-symptomatic individuals. In contrast to antibody responses, population-level surveillance of the T cell response is unlikely to be feasible in the near term. Focused evaluation in specific sub-groups, including vaccine recipients, should be prioritized.. Symptomatic adult COVID-19 cases consistently show peripheral T cell lymphopenia, which positively correlates with increased disease severity, duration of RNA positivity, and non-survival; while asymptomatic and paediatric cases display preserved counts. People with severe or critical disease generally develop more robust, virus-specific T cell responses. T cell memory and effector function has been demonstrated against multiple viral epitopes, and, cross-reactive T cell responses have been demonstrated in unexposed and uninfected adults, but the significance for protection and susceptibility, respectively, remains unclear”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7833159/
145) Galais et al.: Intrafamilial Exposure to SARS-CoV-2 Associated with Cellular Immune Response without Seroconversion, France. CDC, Emerging Infectious Diseases, Volume 27, Number 1 – January 2021.
”Overall, our results indicate that persons exposed to SARS-CoV-2 may develop virus-specific T-cell responses without detectable circulating antibodies. This aspect of the immune response against SARS-CoV-2 contributes substantially to the understanding of the natural history of COVID-19. Furthermore, our data indicate that epidemiologic data relying solely on the detection of SARS-CoV-2 antibodies may lead to a substantial underestimation of prior exposure to the virus”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7774579/
146) J. Dan et al.: Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science, 2021 Jan 6: eabf4063
”Individual case reports show that reinfections with SARS-CoV-2 are occurring (76, 77). However, a 2,800 person study found no symptomatic re-infections over a ~118 day window (78), and a 1,246 person study observed no symptomatic re-infections over 6 months (79). We observed heterogeneity in the magnitude of adaptive immune responses to SARS-CoV-2 persisting into the immune memory phase. It is therefore possible that a fraction of the SARS-CoV-2-infected population with low immune memory would become susceptible to re-infection relatively soon. While gender and disease severity both contribute some to the heterogeneity of immune memory reported here, the source of much of the heterogeneity in immune memory to SARS-CoV-2 is unknown and worth further examination. Perhaps heterogeneity derives from low cumulative viral load or a small initial inoculum in some individuals. Nevertheless, our data show immune memory in at least three immunological compartments was measurable in ~95% of subjects 5 to 8 months PSO, indicating that durable immunity against secondary COVID-19 disease is a possibility in most individuals”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7919858/
147) Tan et al.: Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Reports, 2021 Feb 9; 34(6): 108728.
”These findings provide support for the prognostic value of early functional SARS-CoV-2-specific T cells with important implications in vaccine design and immune monitoring… Although rapid induction and quantity of humoral responses associate with an increase in disease severity, early induction of interferon (IFN)-γ-secreting
SARS-CoV-2-specific T cells is present in patients with mild disease and accelerated viral clearance”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7826084/
148) Gaebler et al.: Evolution of antibody immunity to SARS-CoV-2. Nature, 2021 Mar; 591(7851): 639–644.
“We conclude that the memory B cell response to SARS-CoV-2 evolves between 1.3 and 6.2 months after infection in a manner that is consistent with antigen persistence”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8221082/
149) DiPiazza et al.: T cell immunity to SARS-CoV-2 following natural infection and vaccination. Biochemical and Biophysical Research Communications, 2021 Jan 29; 538: 211–217
“Although T cell durability to SARS-CoV-2 remains to be determined, current data and past experience from human infection with other CoVs demonstrate the potential for persistence and the capacity to control viral replication and host disease”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7584424/
150) Newell et al.: Switched and unswitched memory B cells detected during SARS-CoV-2 convalescence correlate with limited symptom duration. PLOS One, 2021; 16(1): e0244855.
”We observed a significant negative correlation between the frequency of peripheral blood memory B cells and the duration of symptoms for convalescent subjects. Memory B cell subsets in convalescent subjects were composed of classical CD24+ class-switched memory B cells, but also activated CD24-negative and natural unswitched CD27+ IgD+ IgM+ subsets. Memory B cell frequency was significantly correlated with both IgG1 and IgM responses to the SARS-CoV-2 spike protein receptor binding domain (RBD) in most seropositive subjects. IgM+ memory, but not switched memory, directly correlated with virus-specific antibody responses, and remained stable over 3 months. Our findings suggest that the frequency of memory B cells is a critical indicator of disease resolution, and that IgM+ memory B cells may play an important role in SARS-CoV-2 immunity”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7843013/
151) J. Dan et al.: Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science, 2021 Jan 6: eabf4063.
“Substantial immune memory is generated after COVID-19, involving all four major types of immune memory. About 95% of subjects retained immune memory at ~6 months after infection. Circulating antibody titers were not predictive of T cell memory. Thus, simple serological tests for SARS-CoV-2 antibodies do not reflect the richness and durability of immune memory to SARS-CoV-2. This work expands our understanding of immune memory in humans. These results have implications for protective immunity against SARS-CoV-2 and recurrent COVID-19”.
Notably, memory B cells specific for the Spike protein or RBD were detected in almost all COVID-19 cases, with no apparent half-life at 5 to 8 months post-infection. Other studies of RBD memory B cells are reporting similar findings (50, 60). B cell memory to some other infections has been observed to be long-lived, including 60+ years after smallpox vaccination (61), or 90+ years after infection with influenza”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7919858/
https://www.science.org/doi/10.1126/science.abf4063
152) Wajnberg et al.: Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science, 2020 Dec 4; 370(6521): 1227–1230
“Wajnberg et al. used a cohort of more than 30,000 infected individuals with mild to moderate COVID-19 symptoms to determine the robustness and longevity of the anti–SARS-CoV-2 antibody response. They found that neutralizing antibody titers against the SARS-CoV-2 spike protein persisted for at least 5 months after infection. Although continued monitoring of this cohort will be needed to confirm the longevity and potency of this response, these preliminary results suggest that the chance of reinfection may be lower than is currently feared”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7810037/
153) Hanrath et al.: Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. Journal of Infection, 2020 Dec 26.
“Immunity to seasonal coronaviruses is maintained for up to 12 months. Recent unpublished studies in HCWs reported no evidence of symptomatic reinfection, suggesting that immunity is maintained for at least 6 months. In these studies there was a relatively low event rate (123 and 20 symptomatic infections respectively). It is possible that the protective immunity observed may have been explained in part by the relatively low incidence of SARS-CoV-2 infection. Our data confirms and extends these findings. Despite 290 symptomatic infections in 10,137 non-immune HCWs, there were no symptomatic reinfections in over 1000 HCWs with past infection. We conclude that SARS-CoV-2 infection appears to result in protection against symptomatic infection in working age adults, at least in the short term. This is consistent with the low rates of reinfection reported in the literature”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7832116/
154) M. Hellerstein: What are the roles of antibodies versus a durable, high quality T-cell response in protective immunity against SARS-CoV-2? Vaccine: X, 2020 Dec 11; 6: 100076.
“Progress in laboratory markers for SARS-CoV2 has been made with identification of epitopes on CD4 and CD8 T-cells in convalescent blood. These are much less dominated by spike protein than in previous coronavirus infections. Although most vaccine candidates are focusing on spike protein as antigen, natural infection by SARS-CoV-2 induces broad epitope coverage, cross-reactive with other betacoronviruses”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7452821/
155) Hartley et al.:
Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. ScienceImmunology, 2020 Dec 22; 5(54): eabf8891
“Rapid decline of anti-SARS-CoV-2 serum IgG levels beyond 20 days post-diagnosis and the transient presence of circulating plasmablasts have led to questions about the longevity of immunity (17–21). In contrast, antigen-specific memory T cells and memory B (Bmem) cells can be detected in convalescence (22–24).
As these memory cells are programmed to respond rapidly upon subsequent antigen encounter, it is reasonable to hypothesize that these long-lived memory cells provide durable long-term immunity (4, 25). However, detailed insight into the nature and longevity of the Bmem cell compartment specific to SARS-CoV-2 is currently still unresolved (26)…
COVID-19 patients rapidly generate B cell memory to both the spike and nucleocapsid antigens following SARS-CoV-2 infection…
The fact that nearly all RBD-specific IgG+ Bmem cells expressed CD27 and their numbers correlated with circulating TFH cells is indicative of long-lived immune memory…
Several studies have reported a decrease in B cell frequencies during COVID-19 infection which normalized in convalescence (31–34). As these studies report frequencies rather than absolute numbers, the reported decrease might have been confounded by an increase in other immune subsets such as NK cells (31, 33)….
Our results indicate that SARS-CoV-2 infection generates long-lasting B cell memory up to 8 months post-infection that could be protective against systemic disease upon reinfection.
In summary, RBD- and NCP-specific IgG and Bmem cells were detected in all 25 patients with a history of COVID-19. While specific IgG levels in serum declined with time post-symptom onset, SARS-CoV-2-specific Bmem cell numbers persisted, and RBD-specific Bmem cell numbers correlated with TFH cell numbers. This long-term presence of circulating Bmem cell subsets directed against both the major SARS-CoV-2 neutralization target (RBD) and a non-neutralizing target (NCP) is indicative of persisting immune memory following natural exposure and could provide a means to evaluate protection from severe COVID-19 following vaccination”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7877496/
156) Bernardes et al.: Longitudinal Multi-omics Analyses Identify Responses of Megakaryocytes, Erythroid Cells, and Plasmablasts as Hallmarks of Severe COVID-19. Immunity,
2020 Dec 15; 53(6): 1296–1314.e9.
“The study demonstrates broad cellular effects of SARS-CoV-2 infection beyond adaptive immune cells…
In summary, we observed an increase in B cell clonality in COVID-19, and there was an increase of memory B cells and PBs, dominated by the IgA and IgG isotypes and a skewed use of the IGHV gene early during the disease course”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7689306/
157) Lumley et al.: Antibodies to SARS-CoV-2 are associated with protection against reinfection.
MedRxiv, doi: https://doi.org/10.1101/2020.11.18.20234369 (pre-print)
“Critical to understand whether infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) protects from subsequent reinfection… 12219 HCWs participated…prior SARS-CoV-2 infection that generated antibody responses offered protection from reinfection for most people in the six months following infection.”
https://www.medrxiv.org/content/10.1101/2020.11.18.20234369v1
158) Ripperger et al.: Orthogonal SARS-CoV-2 Serological Assays Enable Surveillance of Low-Prevalence Communities and Reveal Durable Humoral Immunity. Immunity, 2020 Nov 17;53(5):925-933.e4
“We conducted a serological study to define correlates of immunity against SARS-CoV-2. Compared to those with mild coronavirus disease 2019 (COVID-19) cases, individuals with severe disease exhibited elevated virus-neutralizing titers and antibodies against the nucleocapsid (N) and the receptor binding domain (RBD) of the spike protein. Age and sex played lesser roles.
We conclude that neutralizing antibodies are stably produced for at least 5-7 months after SARS-CoV-2 infection”.
https://pubmed.ncbi.nlm.nih.gov/33129373/
159) Beretta et al.: Is Cross-Reactive Immunity Triggering COVID-19 Immunopathogenesis? Frontiers in Immunology, 2020; 11: 567710.
”There is also evidence that pre-existing T cell immunity to common cold coronaviruses can prime the response to SARS-CoV-2”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7594548/
160) Faulkner et al.: Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science,
2020 Dec 11; 370(6522): 1339–1343.
“Our results from multiple independent assays demonstrate the presence of preexisting antibodies recognizing SARS-CoV-2 in uninfected individuals… SARS-CoV-2 spike glycoprotein (S)–reactive antibodies were detectable using a flow cytometry–based method in SARS-CoV-2–uninfected individuals and were particularly prevalent in children and adolescents”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7857411/
161) Yang et al.: Naturally activated adaptive immunity in COVID-19 patients. Journal of Cellular and Molecular Medicine,
2020 Nov;24(21):12457-12463
”We demonstrated that the T and B cells are activated naturally and are functional during SARS-CoV-2 infection. These data provide evidence that the adaptive immunity in most patients could be primed to induce a significant immune response against SARS-CoV-2 infection upon receiving standard medical care”.
https://pubmed.ncbi.nlm.nih.gov/32975374/
162) Peng et al.: Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nature Immunology, 2020 Nov 1; 21(11): 1336–1345.
“We studied T cell memory in 42 patients following recovery from COVID-19 (28 with mild disease and 14 with severe disease)…
The identification of T cell responses associated with milder disease will support an understanding of protective immunity.
The breadth and magnitude of T cell responses were significantly higher in severe compared with mild cases.
Broad, and frequently strong, SARS-CoV-2-specific CD4+ and CD8+ T cell responses were seen in the majority of convalescent patients, with significantly larger overall T cell responses in those who had severe compared with mild disease. However, there was a greater proportion of CD8+ T cell compared with CD4+ T cell responses in mild cases, with higher frequencies of multi-cytokine production by matrix (M)- and NP- specific CD8+ T cells”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7611020/
163) Rodda et al.: Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell, 2021 Jan 7;184(1):169-183.e17
“Our data further reveal that SARS-CoV-2-specific IgG memory B cells increased over time. Additionally, SARS-CoV-2-specific memory lymphocytes exhibited characteristics associated with potent antiviral function: memory T cells secreted cytokines and expanded upon antigen re-encounter, whereas memory B cells expressed receptors capable of neutralizing virus when expressed as monoclonal antibodies. Therefore, mild COVID-19 elicits memory lymphocytes that persist and display functional hallmarks of antiviral immunity”.
https://pubmed.ncbi.nlm.nih.gov/33296701/
164) Moderbacher et al.: Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell, 2020 Nov 12; 183(4): 996–1012.e19.
“We completed a combined examination of all three branches of adaptive immunity at the level of SARS-CoV-2-specific CD4+ and CD8+ T cell and neutralizing antibody responses in acute and convalescent subjects. SARS-CoV-2-specific CD4+ and CD8+ T cells were each associated with milder disease. Coordinated SARS-CoV-2-specific adaptive immune responses were associated with milder disease, suggesting roles for both CD4+ and CD8+ T cells in protective immunity in COVID-19. Notably, coordination of SARS-CoV-2 antigen-specific responses was disrupted in individuals ≥ 65 years old. Scarcity of naive T cells was also associated with aging and poor disease outcomes. A parsimonious explanation is that coordinated CD4+ T cell, CD8+ T cell, and antibody responses are protective, but uncoordinated responses frequently fail to control disease, with a connection between aging and impaired adaptive immune responses to SARS-CoV-2”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC3782116/
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7494270/
165) Saini et al.: SARS-CoV-2 genome-wide mapping of CD8 T cell recognition reveals strong immunodominance and substantial CD8 T cell activation in COVID-19 patients. SCIENCE IMMUNOLOGY, 2 Apr 2021, Vol 6, Issue 58.
“COVID-19 patients showed strong T cell responses, with up to 25% of all CD8+ lymphocytes specific to SARS-CoV-2-derived immunodominant epitopes, derived from ORF1 (open reading frame 1), ORF3, and Nucleocapsid (N) protein. A strong signature of T cell activation was observed in COVID-19 patients, while no T cell activation was seen in the ‘non-exposed’ and ‘high exposure risk’ healthy donors”.
https://www.biorxiv.org/content/10.1101/2020.10.19.344911v1?rss=1%22
”The phenotype of the SARS-CoV-2–specific CD8+ T cells revealed a strong T cell activation in patients with COVID-19, whereas minimal T cell activation was seen in healthy individuals. We found that patients with severe disease displayed significantly larger SARS-CoV-2–specific T cell populations compared with patients with mild diseases, and these T cells displayed a robust activation profile. These results further our understanding of T cell immunity to SARS-CoV-2 infection and hypothesize that strong antigen-specific T cell responses are associated with different disease outcomes”.
https://www.science.org/doi/10.1126/sciimmunol.abf7550
https://pubmed.ncbi.nlm.nih.gov/33853928/
166) Mateus et al.: Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science, 2020 Oct 2; 370(6512): 89–94.
”Mateus et al. found that the preexisting reactivity against SARS-CoV-2 comes from memory T cells and that cross-reactive T cells can specifically recognize a SARS-CoV-2 epitope as well as the homologous epitope from a common cold coronavirus. These findings underline the importance of determining the impacts of preexisting immune memory in COVID-19 disease severity”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7574914/
167) P. Doshi.: Covid-19: Do many people have pre-existing immunity? British Medical Journal, 2020 Sep 17;370:m3563
“Six studies have reported T cell reactivity against SARS-CoV-2 in 20% to 50% of people with no known exposure to the virus… in a study of donor blood specimens obtained in the US between 2015 and 2018, 50% displayed various forms of T cell reactivity to SARS-CoV-2… Researchers are also confident that they have made solid inroads into ascertaining the origins of the immune responses. “Our hypothesis, of course, was that it’s so called ‘common cold’ coronaviruses, because they’re closely related…we have really shown that this is a true immune memory and it is derived in part from common cold viruses.”
https://www.bmj.com/content/370/bmj.m3563.long
https://pubmed.ncbi.nlm.nih.gov/32943427/
168) Peng et al.: Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nature immunology, 2020 Nov 1; 21(11): 1336–1345.
“Taken together, this study has demonstrated strong and broad SARS-CoV-2-specific CD4+ and CD8+ T cell responses in the majority of humans who had recovered from COVID-19”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7611020/
169) Jones et al.: Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Nature Immunology, 2020 Nov 1; 21(11): 1336–1345.
”We measured plasma and/or serum antibody responses to the receptor-binding domain (RBD) of the spike (S) protein of SARS-CoV-2 in 343 North American patients infected with SARS-CoV-2 (of which 93% required hospitalization) up to 122 days after symptom onset and compared them to responses in 1548 individuals whose blood samples were obtained prior to the pandemic..
IgG antibodies persisted at detectable levels in patients beyond 90 days after symptom onset, and seroreversion was only observed in a small percentage of individuals… The concentration of these anti-RBD IgG antibodies was also highly correlated with pseudovirus NAb titers, which also demonstrated minimal decay. The observation that IgG and neutralizing antibody responses persist is encouraging, and suggests the development of robust systemic immune memory in individuals with severe infection”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7857394/
170) Cox et al.: Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nature Rewies Immunology, 2020; 20(10): 581–582.
“Recent reports that antibodies to SARS-CoV-2 are not maintained in the serum following recovery from the virus have caused alarm. However, the absence of specific antibodies in the serum does not necessarily mean an absence of immune memory”.
COVID-19 is caused by infection with SARS-CoV-2, which is a member of the coronavirus family. There are currently four human coronaviruses (HCoVs) that cause respiratory infections or the ‘common cold’ (namely, 229E, NL63, OC43 and HKU1), as well as three coronaviruses that have arisen through zoonosis and cause severe diseases in humans, namely, SARS-CoV, MERS-CoV and SARS-CoV-2, which emerged in 2003, 2012 and 2019, respectively…
A recent report suggesting that antibodies to the virus may only be maintained for 2 months has caused speculation that ‘immunity’ to the virus may not be long lived. Similarly, a rapid decline in antibodies was reported in mild cases. It is important to remember that memory B cells and T cells may be maintained even if there are not measurable levels of serum antibodies…
Although spike-specific CD4+ T cells are found in patients with COVID-19, 30–50% of healthy people with no detectable COVID-19 infection also had SARS-CoV-2-specific CD4+ T cells and 20% had CD8+ cytotoxic T cells. These T cells are probably cross-reactive with other HCoVs, but whether they can provide protection from COVID-19 disease remains to be determined.
Studies of patients who became infected with SARS-CoV in 2003 suggested that the infection induced durable T cell responses lasting for 6 years but no long-term memory B cells. Importantly, these T cells were shown to cross-react with the SARS-CoV-2 virus 17 years later”.
https://www.nature.com/articles/s41577-020-00436-4
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7443809/
171) P. Nguyen-Contant et al.: S Protein-Reactive IgG and Memory B Cell Production after Human SARS-CoV-2 Infection Includes Broad Reactivity to the S2 Subunit. American Society for Microbiology ASM, 2020 Sep-Oct; 11(5): e01991-20
“Most importantly, we demonstrate that SARS-CoV-2 infection generates both IgG and IgG MBCs reactive to the novel RBD and the conserved S2 subunit of the S protein…. In conclusion, our analysis investigated Ab and MBC immunity to SARS-CoV-2 in unexposed subjects and individuals soon after recovery from SARS-CoV-2 infection. The findings emphasized the novelty of the SARS-CoV-2 S protein RBD in unexposed subjects. However, IgG reactive to the S2 was widespread in unexposed subjects and likely resulted from exposure to HCoVs. Although our approach was unable to directly identify S2-reactive MBCs in the unexposed subjects, we suggest that these cells were present and strongly contributed S2-reactive IgG early in the response to SARS-CoV-2 infection. The IgG response in convalescent SARS-CoV-2 subjects was also strong against the RBD and, less consistently, against the N protein. Importantly, the convalescent SARS-CoV-2 subjects had generated RBD-reactive and S2-reactive IgG MBCs. The RBD-reactive MBCs are likely to provide strong long-term protection if RBD-reactive neutralizing Ab levels wane and reinfection occurs”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7520599/
172) Gudbjartsson et al.: Humoral Immune Response to SARS-CoV-2 in Iceland. New England Journal of Medicine, 2020 Oct 29;383(18):1724-173
”Our results indicate that antiviral antibodies against SARS-CoV-2 did not decline within 4 months after diagnosis. We estimate that the risk of death from infection was 0.3%”.
https://pubmed.ncbi.nlm.nih.gov/32871063/
173) Juno et al.: Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nature Medicine, 2020 Sep;26(9):1428-1434
”We found that S-specific antibodies, memory B cells and cTFH are consistently elicited after SARS-CoV-2 infection, demarking robust humoral immunity and positively associated with plasma neutralizing activity.
https://pubmed.ncbi.nlm.nih.gov/32661393/
174) Isho et al.: Evidence for sustained mucosal and systemic antibody responses to SARS-CoV-2 antigens in COVID-19 patients.
MedRxiv, August 04, 2020,
doi: https://doi.org/10.1101/2020.08.01.20166553 (pre-print)
“Whereas anti-CoV-2 IgA antibodies rapidly decayed, IgG antibodies remained relatively stable up to 115 days PSO in both biofluids. Importantly, IgG responses in saliva and serum were correlated, suggesting that antibodies in the saliva may serve as a surrogate measure of systemic immunity”.
https://www.medrxiv.org/content/10.1101/2020.08.01.20166553v1
175) T. Hicklin, Immune cells for common cold may recognize SARS-CoV-2. National Institute of Health NIH, August 18, 2020 (scientific article)
“Now a new study led by scientists at La Jolla Institute for Immunology (LJI) shows that memory helper T cells that recognize common cold coronaviruses also recognize matching sites on SARS-CoV-2, the virus that causes COVID-19. The research, published Aug. 4, 2020 in Science, may explain why some people have milder COVID-19 cases than others—though the researchers emphasize that this is speculation and much more data is needed.
“We have now proven that, in some people, pre-existing T cell memory against common cold coronaviruses can cross-recognize SARS-CoV-2, down to exact molecular structures,” says LJI Research Assistant Professor Daniela Weiskopf, Ph.D., who co-led the new study with LJI Professor Alessandro Sette, Dr. Biol. Sci. “This could help explain why some people show milder symptoms of disease while others get severely sick.”
https://www.lji.org/news-events/news/post/exposure-to-common-cold-coronaviruses-can-teach-the-immune-system-to-recognize-sars-cov-2/
https://www.nih.gov/news-events/nih-research-matters/immune-cells-common-cold-may-recognize-sars-cov-2
176) Braun et al.: SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature, 2020 Nov;587(7833):270-274
“Spike-protein-reactive T cell lines generated from SARS-CoV-2-naive healthy donors responded similarly to the C-terminal region of the spike proteins of the human endemic coronaviruses 229E and OC43, as well as that of SARS-CoV-2. This results indicate that spike-protein cross-reactive T cells are present, which were probably generated during previous encounters with endemic coronaviruses… the presence of spike-protein cross-reactive T cells in a considerable fraction of the general population may affect the dynamics of the current pandemic”.
https://www.nature.com/articles/s41586-020-2598-9
https://pubmed.ncbi.nlm.nih.gov/32726801/
177) Sette et al.: Pre-existing immunity to SARS-CoV-2: the knowns and unknowns. Nature Rewies Immunology, 2020 Aug;20(8):457-458
“T cell reactivity against SARS-CoV-2 was observed in unexposed people… it is speculated that this reflects T cell memory to circulating ‘common cold’ coronaviruses”.
https://pubmed.ncbi.nlm.nih.gov/32636479/
Fulltext:
“In conclusion, it is now established that SARS-CoV-2 pre-existing immune reactivity exists to some degree in the general population. It is hypothesized, but not yet proven, that this might be due to immunity to CCCs. This might have implications for COVID-19 disease severity, herd immunity”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7339790/
178) Bert et al.: SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature, 2020 Aug;584(7821):457-462
“In all of these individuals, we found CD4 and CD8 T cells that recognized multiple regions of the N protein. Next, we showed that patients (n = 23) who recovered from SARS (the disease associated with SARS-CoV infection) possess long-lasting memory T cells that are reactive to the N protein of SARS-CoV 17 years after the outbreak of SARS in 2003; these T cells displayed robust cross-reactivity to the N protein of SARS-CoV-2. We also detected SARS-CoV-2-specific T cells in individuals with no history of SARS, COVID-19 or contact with individuals who had SARS and/or COVID-19 (n = 37).
Thus, infection with betacoronaviruses induces multi-specific and long-lasting T cell immunity against the structural N protein”.
https://www.nature.com/articles/s41586-020-2550-z
https://pubmed.ncbi.nlm.nih.gov/32668444/
179) F. Collins: Immune T Cells May Offer Lasting Protection Against COVID-19. National institute of Health, Blog, July 28th, 2020
“Much of the study on the immune response to SARS-CoV-2, the novel coronavirus that causes COVID-19, has focused on the production of antibodies. But, in fact, immune cells known as memory T cells also play an important role in the ability of our immune systems to protect us against many viral infections, including—it now appears—COVID-19. An intriguing new study of these memory T cells suggests they might protect some people newly infected with SARS-CoV-2 by remembering past encounters with other human coronaviruses. This might potentially explain why some people seem to fend off the virus and may be less susceptible to becoming severely ill with COVID-19.”
https://directorsblog.nih.gov/2020/07/28/immune-t-cells-may-offer-lasting-protection-against-covid-19/
180) A. Grifoni et al.: Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals.
Cell, 2020 Jun 25; 181(7): 1489–1501.e15.
”Using HLA class I and II predicted peptide “megapools,” circulating SARS-CoV-2-specific CD8+ and CD4+ T cells were identified in ∼70% and 100% of COVID-19 convalescent patients.
Importantly, we detected SARS-CoV-2-reactive CD4+ T cells in ∼40%–60% of unexposed individuals, suggesting cross-reactive T cell recognition between circulating “common cold” coronaviruses and SARS-CoV-2”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7237901/
181) Robiani et al.: Convergent Antibody Responses to SARS-CoV-2 in Convalescent Individuals. Nature. 2020 Aug; 584(7821): 437–442.
“Antibody sequencing revealed expanded clones of RBD-specific memory B cells expressing closely related antibodies in different individuals. Despite low plasma titers, antibodies to three distinct epitopes on RBD neutralized at half-maximal inhibitory concentrations (IC50s) as low as single digit ng/mL. Thus, most convalescent plasmas obtained from individuals who recover from COVID-19 do not contain high levels of neutralizing activity. Nevertheless, rare but recurring RBD-specific antibodies with potent antiviral activity were found in all individuals tested”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7442695/
182) Ling Ni et al.:
Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity, 2020 Jun 16; 52(6): 971–977.e3.
“Collected blood from COVID-19 patients who have recently become virus-free, and therefore were discharged, and detected SARS-CoV-2-specific humoral and cellular immunity in eight newly discharged patients. Follow-up analysis on another cohort of six patients 2 weeks post discharge also revealed high titers of immunoglobulin G (IgG) antibodies. In all 14 patients tested, 13 displayed serum-neutralizing activities in a pseudotype entry assay. Notably, there was a strong correlation between neutralization antibody titers and the numbers of virus-specific T cells. Our work provides a basis for further analysis of protective immunity to SARS-CoV-2, and understanding the pathogenesis of COVID-19, especially in the severe cases”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7196424/
183) Wang et al.: Neutralizing Antibodies Responses to SARS-CoV-2 in COVID-19 Inpatients and Convalescent Patients. Clinical infectious Diseases, 2020 Jun 4 : ciaa721.
”The neutralizing antibodies were detected even at the early stage of disease, and a significant response showed in convalescent patients”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7314147/
184) Weiskopf et al.: Phenotype of SARS-CoV-2-specific T-cells in COVID-19 patients with acute respiratory distress syndrome. SCIENCE IMMUNOLOGY, 26 Jun 2020 Vol 5, Issue 48
“We detected SARS-CoV-2-specific CD4+ and CD8+ T cells in 100% and 80% of COVID-19 patients, respectively. We also detected low levels of SARS-CoV-2-reactive T-cells in 20% of the healthy controls, not previously exposed to SARS-CoV-2 and indicative of cross-reactivity due to infection with ‘common cold’ coronaviruses”.
https://www.medrxiv.org/content/10.1101/2020.04.11.20062349v2?ijkey=747f090d21ac4834150ea548be0f6628059c1053&keytype2=tf_ipsecsha
https://www.science.org/doi/10.1126/sciimmunol.abd2071
185) Smetana et al.: Decreasing Seroprevalence of Measles Antibodies after Vaccination – Possible Gap in Measles Protection in Adults in the Czech Republic.
PLOS One, 2017 Jan 13;12(1): e0170257.
”A long term high rate of seropositivity persists after natural measles infection. By contrast, it decreases over time after vaccination”.
https://pubmed.ncbi.nlm.nih.gov/28085960/
186) A. Chia et al.: Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine, 2016 Apr 12; 34(17): 2008–2014.
“Severe acute respiratory syndrome (SARS) is a highly contagious infectious disease which first emerged in late 2002, caused by a then novel human coronavirus, SARS coronavirus… In this study, the screening for the presence of SARS-specific T cells in a cohort of three SARS-recovered individuals at 9 and 11 years post-infection was carried out, and all memory T cell responses detected target the SARS-CoV structural proteins. Two CD8+ T cell responses targeting the SARS-CoV membrane (M) and nucleocapsid (N) proteins were characterized by determining their HLA restriction and minimal T cell epitope regions. Furthermore, these responses were found to persist up to 11 years post-infection… The knowledge of the persistence of SARS-specific celullar immunity targeting the viral structural proteins in SARS-recovered individuals is important…”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7115611/
187) Shridhar et al.: Cellular immune correlates of protection against symptomatic pandemic influenza. Nature Medicine, 2013 Oct;19(10):1305-12
“The 2009 H1N1 pandemic (pH1N1) provided a unique natural experiment to determine whether crossreactive cellular immunity limits symptomatic illness in antibody-naive individuals… Higher frequencies of pre-existing T cells to conserved CD8 epitopes were found in individuals who developed less severe illness, with total symptom score having the strongest inverse correlation with the frequency of interferon-γ (IFN-γ)(+) interleukin-2 (IL-2)(-) CD8(+) T cells (r = -0.6, P = 0.004)… CD8(+) T cells specific to conserved viral epitopes correlated with crossprotection against symptomatic influenza.”
https://pubmed.ncbi.nlm.nih.gov/24056771/
188) Petrova et al.: Cross-Reactivity of T Cells and Its Role in the Immune System. Critical Reviews in Immunology. 2012; 32(4): 349–372.
“T-cell cross-reactivity based on recognition of more than one peptide by a TCR can be a very important facet of immune responses. Networks of cross-reactive T cells might generate a robust system that can deal with most novel pathogens in the absence of large-scale de novo production of naïve T cells, as is found in human post-thymic involution”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC3595599/
189) Wilkinson et al.: Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nature Medicine, 2012 Jan 29;18(2):274-80
“Protective immunity against influenza virus infection is mediated by neutralizing antibodies, but the precise role of T cells in human influenza immunity is uncertain. We conducted influenza infection studies in healthy volunteers with no detectable antibodies to the challenge viruses H3N2 or H1N1. We mapped T cell responses to influenza before and during infection. We found a large increase in influenza-specific T cell responses by day 7, when virus was completely cleared from nasal samples and serum antibodies were still undetectable. Preexisting CD4+, but not CD8+, T cells responding to influenza internal proteins were associated with lower virus shedding and less severe illness. These CD4+ cells also responded to pandemic H1N1 (A/CA/07/2009) peptides and showed evidence of cytotoxic activity. These cells are an important statistical correlate of homotypic and heterotypic response and may limit severity of influenza infection by new strains in the absence of specific antibody responses”.
https://pubmed.ncbi.nlm.nih.gov/22286307/
190) Tang et al.: Lack of Peripheral Memory B Cell Responses in Recovered Patients with Severe Acute Respiratory Syndrome: A Six-Year Follow-Up Study, The Journal of immunology, June 15, 2011, 186 (12) 7264-7268;
“In conclusion, specific IgG Ab to SARS-CoV eventually vanish in most SARS patients. Peripheral memory B cell responses are undetectable in all the patients. However, quite a portion of the patients maintain T cell memory even 6 y postinfection. Whether the T cell anamnestic response is adequate to protect a person from reinfection requires further investigation”.
https://www.jimmunol.org/content/186/12/7264
https://pubmed.ncbi.nlm.nih.gov/21576510/
191) Vivier et al.: Innate or adaptive immunity? The example of natural killer cells. Science, 2011 Jan 7;331(6013):44-9
“More recently, a more nuanced view of NK cells has emerged. NK cells are now recognized to express a repertoire of activating and inhibitory receptors that is calibrated to ensure self-tolerance while allowing efficacy against assaults such as viral infection and tumor development. Moreover, NK cells do not react in an invariant manner but rather adapt to their environment. Finally, recent studies have unveiled that NK cells can also mount a form of antigen-specific immunologic memory. NK cells thus exert sophisticated biological functions that are attributes of both innate and adaptive immunity, blurring the functional borders between these two arms of the immune response”.
https://pubmed.ncbi.nlm.nih.gov/21212348/
192) Wrammert et al.: Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. Journal of Experimental Medicine, 2011 Jan 17; 208(1): 181–193.
“The 2009 pandemic H1N1 influenza pandemic demonstrated the global health threat of reassortant influenza strains. Herein, we report a detailed analysis of plasmablast and monoclonal antibody responses induced by pandemic H1N1 infection in humans. Unlike antibodies elicited by annual influenza vaccinations, most neutralizing antibodies induced by pandemic H1N1 infection were broadly cross-reactive against epitopes in the hemagglutinin (HA) stalk and head domain of multiple influenza strains.
The antibodies were from cells that had undergone extensive affinity maturation. Based on these observations, we postulate that the plasmablasts producing these broadly neutralizing antibodies were predominantly derived from activated memory B cells specific for epitopes conserved in several influenza strains…
We propose that the expansion of these rare types of memory B cells may explain why most people did not become severely ill, even in the absence of preexisting protective antibody titers. Recent studies in mice strongly support the idea that consecutive immunizations with antigens from divergent influenza stains can indeed hone the antibody response to preferentially target broadly protective conserved epitopes…
In conclusion, analyses of the 46 mAbs induced by pandemic H1N1 infection indicated frequent activation of broadly reactive B cells. We propose that these cells had a memory cell origin caused by cross-reactivity to conserved and functionally important epitopes”.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC3023136/
193) Bodewes et al.: Yearly influenza vaccinations: a double-edged sword? Bodewes et al. The Lancet Infectious Diseases, Volume 9, Issue 12, December 2009, Pages 784-788
”However, it has been shown-mainly in animals-that infection with influenza A viruses can induce protective immunity to influenza A viruses of other unrelated subtypes. This so-called heterosubtypic immunity does not provide full protection, but can limit virus replication and reduce morbidity and mortality of the host. This type of immunity might be relevant to human beings when a new subtype of influenza A virus is introduced into the population, such as the new influenza A H1N1 virus responsible for the present influenza pandemic and the highly pathogenic avian influenza H5N1 viruses that are causing an ever increasing number of human infections with high mortality rates.
Preventing infection with seasonal influenza viruses by vaccination might prevent the induction of heterosubtypic immunity to pandemic strains, which might be a disadvantage to immunologically naive people-eg, infants”.
https://www.sciencedirect.com/science/article/abs/pii/S1473309909702634
https://pubmed.ncbi.nlm.nih.gov/19879807/
194) Greenbaum et al.: Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. 2009 Dec 1; 106(48): 20365–20370
“A major concern about the ongoing swine-origin H1N1 influenza virus (S-OIV) outbreak is that the virus may be so
different from seasonal H1N1 that little immune protection exists in the human population. Memory T-cell immunity against S-OIV is present in the adult population and that such memory is of similar magnitude as the pre-existing memory against seasonal H1N1 influenza…the conservation of a large fraction of T-cell epitopes suggests that the severity of an S-OIV infection, as far as it is determined by susceptibility of the virus to immune attack, would not differ much from that of seasonal flu.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC2777968/
195) Centers for Disease Control and Prevention (CDC): Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. Morbidity and Mortality weekly Report, 2009 May 22;58(19):521-4.
“No increase in cross-reactive antibody response to the novel influenza A (H1N1) virus was observed among adults aged >60 years. These data suggest that receipt of recent (2005–2009) seasonal influenza vaccines is unlikely to elicit a protective antibody response to the novel influenza A (H1N1) virus”.
https://pubmed.ncbi.nlm.nih.gov/19478718/
196) X. Ju et al.: Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature, 2008 Sep 25;455(7212):532-6
“Here we show that of the 32 individuals tested that were born in or before 1915, each showed seroreactivity with the 1918 virus, nearly 90 years after the pandemic. Seven of the eight donor samples tested had circulating B cells that secreted antibodies that bound the 1918 HA. Thus, these studies demonstrate that survivors of the 1918 influenza pandemic possess highly functional, virus-neutralizing antibodies to this uniquely virulent virus, and that humans can sustain circulating B memory cells to viruses for many decades after exposure—well into the tenth decade of life”.
https://www.nature.com/articles/nature07231
https://pubmed.ncbi.nlm.nih.gov/18716625/
197) Welsh et al.: No one is naive: the significance of heterologous T-cell immunity. Nature Reviewa Immunology, 2002 Jun;2(6):417-26.
”Memory T cells that are specific for one virus can become activated during infection with an unrelated heterologous virus, and might have roles in protective immunity and immunopathology. The course of each infection is influenced by the T-cell memory pool that has been laid down by a host’s history of previous infections, and with each successive infection, T-cell memory to previously encountered agents is modified”.
https://pubmed.ncbi.nlm.nih.gov/12093008/
198) Berczi et al.: Neuroimmunoregulation and natural immunity. Domestic Animal Endocrinilogy, 1998 Sep;15(5):273-81
”Bone marrow activity and leukocyte function are high and the liver is converted to the rapid production of acute-phase proteins (APP). APP include LBP, CRP, fibrinogen, some complement components, enzyme inhibitors, and anti-inflammatory proteins, which may rise in the serum from several hundred to a thousand times within 24-48 hr. Therefore, natural immunity is a polyspecific response to homotopes, which functions as an instantaneous defense mechanism in health and which is rapidly boosted by cytokines and hormones during febrile illness. This is a highly successful defense reaction, as in the overwhelming majority of cases, febrile illness leads to recovery and the development of adaptive immunity in man and higher animals”.
https://pubmed.ncbi.nlm.nih.gov/9785030/
199) Valk et al.: SARS-Cov-2: The Relevance and Prevention of Aerosol Transmission. Journal of Occupational and Environmeltal Medicine. 2021 Jun 1;63(6):e395-e401
“However, the large number of COVID-19 infections cannot be explained only by droplet deposition or contact contamination. It seems very plausible that aerosols are important in transmitting SARS-Cov-2. It has been demonstrated that SARS-CoV-2 remains viable in aerosols for hours, facilitating rapid distribution of the virus over great distances. Aerosols may, therefore, also be responsible for so-called super-spreader events.
https://pubmed.ncbi.nlm.nih.gov/33871953/
200) Hawks et al.: Infectious SARS-CoV-2 Is Emitted in Aerosol Particles. American Society for Microbiology, 2021 Oct 26;12(5): e0252721
“Respiratory viruses such as SARS-CoV-2 are transmitted in respiratory droplets and aerosol particles, which are released during talking, breathing, coughing, and sneezing. Noncontact transmission of SARS-CoV-2 has been demonstrated, suggesting transmission via virus carried through the air…. The majority of virus was contained within particles <5 μm in size. Thus, we provide direct evidence that, in hamsters, SARS-CoV-2 is an airborne virus.
https://pubmed.ncbi.nlm.nih.gov/34663099/
201) Setti et al.: Airborne Transmission Route of COVID-19: Why 2 Meters/6 Feet of Inter-Personal Distance Could Not Be Enough. International Journal of Environmental Research and Public Health, 2020 Apr 23;17(8):2932.
“Recently published studies support the hypothesis of virus transmission over a distance of 2 m from an infected person. Researchers have proved the higher aerosol and surface stability of SARS-COV-2 as compared with SARS-COV-1 (with the virus remaining viable and infectious in aerosol for hours) and that airborne transmission of SARS-CoV can occur besides close-distance contacts. Indeed, there is reasonable evidence about the possibility of SARS-COV-2 airborne transmission due to its persistence into aerosol droplets in a viable and infectious form. Based on the available knowledge and epidemiological observations, it is plausible that small particles containing the virus may diffuse in indoor environments covering distances up to 10 m from the emission sources, thus representing a kind of aerosol transmission”.
https://pubmed.ncbi.nlm.nih.gov/32340347/
202) Gohli et al.: Dispersion of SARS-CoV-2 in air surrounding COVID-19-infected individuals with mild symptoms. Indoor Air, 2022 Feb;32(2): e13001.
“Accumulating evidence suggests that SARS-CoV-2 can be transmitted by aerosols, and not only via larger respiratory droplets”.
https://pubmed.ncbi.nlm.nih.gov/35225394/
203) Bahl et al.: Airborne or Droplet Precautions for Health Workers Treating Coronavirus Disease 2019? The Journal of Infectious Diseases, 2022 May 4;225(9):1561-1568
“Of 10 studies on horizontal droplet distance, 8 showed droplets travel more than 2 meters (≈6 feet), in some cases up to 8 meters. Available studies also show that SARS-CoV-2 can be detected in the air, and remain viable 3 hours after aerosolization”.
https://pubmed.ncbi.nlm.nih.gov/32301491/
204) Port et al.: Increased small particle aerosol transmission of B.1.1.7 compared with SARS-CoV-2 lineage A in vivo. Nature Microbiology, 2022 Feb;7(2):213-223
“The major transmission route for SARS-CoV-2 is airborne. However, previous studies could not elucidate the contribution between large droplets and aerosol transmission of SARS-CoV-2 and its variants… Aerosol transmission of both variants was confirmed in all sentinels after 24 h of exposure as demonstrated by respiratory virus shedding and seroconversion. Productive transmission also occurred after 1 h of exposure, highlighting the efficiency of this transmission route… Interestingly, after donors were infected with a mix of both variants, the Alpha variant outcompeted the lineage A variant in an airborne transmission chain. Overall, these data indicate that a lower infectious dose of the Alpha variant, compared to lineage A, could be sufficient for successful transmission.
https://pubmed.ncbi.nlm.nih.gov/35017676/
205) Mallach et al.: Aerosol SARS-CoV-2 in hospitals and long-term care homes during the COVID-19 pandemic. PLOS One, 2021 Sep 30;16(9):e0258151
“We deployed particulate air samplers in rooms with COVID-19 positive patients in hospital ward and ICU rooms, rooms in long-term care homes experiencing outbreaks, and a correctional facility experiencing an outbreak. Samplers were placed between 2 and 3 meters from the patient. Aerosol (small liquid particles suspended in air) samples were collected onto gelatin filters by Ultrasonic Personal Air Samplers… In total, 138 samples were collected from 99 rooms. RNA samples were positive in 9.1% (6/66) of samples obtained with the UPAS 2.5μm samplers, 13.5% (7/52) with the UPAS 10μm samplers, and 10.0% (2/20) samples obtained with the Coriolis samplers. Viral RNA was detected in 15.1% of the rooms sampled. There was no significant difference in viral RNA recovery between the different room locations or samplers. Method development experiments indicated minimal loss of SARS-CoV-2 viability via the personal air sampler operation”.
https://pubmed.ncbi.nlm.nih.gov/34591919/
206) Tang et al.: Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environmental International, 2020 Nov; 144: 106039
“We aimed to review the evidence of aerosol transmission of SARS-CoV-2. Several studies support that aerosol transmission of SARS-CoV-2 is plausible, and the plausibility score (weight of combined evidence) is 8 out of 9”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7413047/
207) Doremalen et al.: Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. The New England Journal of Medicine, 2020 Mar 17: NEJMc2004973.
“Our results indicate that aerosol and fomite transmission of SARS-CoV-2 is plausible, since the virus can remain viable and infectious in aerosols for hours and on surfaces up to days (depending on the inoculum shed)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7121658/
208) Jayaweera et al.: Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environmental Research, 020 Sep; 188: 109819
“The case studies found worldwide indicate that the behavior of the SARS-CoV-2 virus has been unprecedentedly unique with more survival and viable rates in the air and believed to linger in the air for an extended period.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7293495/
209) Chatterjee et al.: How coronavirus survives for hours in aerosols. Physics of Fluids, 2021 Aug;33(8):081708
“Recently published titer measurements of SARS-CoV-2 in aerosols have disclosed that the coronavirus can survive for hours”.
https://pubmed.ncbi.nlm.nih.gov/34471334/
210) M C Jarvis: Aerosol Transmission of SARS-CoV-2: Physical Principles and Implications. Frontiers in Public Health, 2020 Nov 23;8:590041
“Evidence has emerged that SARS-CoV-2, the coronavirus that causes COVID-19, can be transmitted airborne in aerosol particles as well as in larger droplets or by surface deposits.
There are also opportunities to inactivate SARS-CoV-2 in aerosol form with sunlight or UV lamps”.
https://pubmed.ncbi.nlm.nih.gov/33330334/
211) Finch et al.: SARS-CoV-2 antibodies protect against reinfection for at least 6 months in a multicentre seroepidemiological workplace cohort. PLOS Biology Collection – Antiviral innate Immunity, 2022 Feb 10;20(2):e3001531
“The presence of SARS-CoV-2 antibodies at baseline is associated with around 72% to 86% reduced odds of a subsequent PCR positive test based on our point estimates. This suggests that primary infection with SARS-CoV-2 provides protection against reinfection in the majority of individuals, at least over a 6-month time period”.
https://pubmed.ncbi.nlm.nih.gov/35143473/
212) Flacco et al.: Rate of reinfections after SARS-CoV-2 primary infection in the population of an Italian province: a cohort study. Journal of Public Health, Oxford Academics, 2021 Sep 8 : fdab346.
This study confirms previous findings on a low risk of SARS-CoV-2 reinfection. If confirmed, these findings suggest that more targeted restriction policies can be applied to the subjects that recovered after a first infection. Overall, these findings are in line with the available literature,3–6,8 suggesting that the natural immunity confers substantial protection in the first months after the primary infection: all the published cohort studies reported reinfection rates lower than 1%,3–6 and the only Italian study, on a sample of 1579 positive subjects, reported a virtually identical reinfection rate (0.32%).6 However, this is the first study with a follow-up longer than 12 months, and the first to provide data separately for the minors: notably, no reinfections were recorded after 12 months of follow-up, and none of the 832 included minors experienced a reinfection.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8522392/
213) Deadline, DR tv: Thea Kølsen Fischer, professor of public health, viral infections and epidemics at the University of Copenhagen, head of research at Nordsjællands Hospital, former head of the national virus surveillance at the Statens Serum Institut and trained disease detective at the Centers for Disease Control in the USA: ”80% get no or only mild symptoms with this virus”.
Source: DR tv, Deadline 23.11.2021. 22.50 minute. (Danish speak)
214) Andeweg et al. Elevated risk of infection with SARS-CoV-2 Beta, Gamma, and Delta variant compared to Alpha variant in vaccinated individuals. Science Translational Medicine, 2022 Jul 21; eabn4338.
”We analyzed 28,578 sequenced SARS-CoV-2 samples from individuals with known immune status obtained through national community testing in the Netherlands from March to August 2021. We found evidence of an increased risk of infection by the Beta (B.1.351), Gamma (P.1), or Delta (B.1.617.2) variants compared to the Alpha (B.1.1.7) variant after vaccination. No clear differences were found between vaccines. However, the effect was larger in the first 14-59 days after complete vaccination compared to ≥60 days.
In contrast to vaccine-induced immunity, there was no increased risk for re-infection with Beta, Gamma or Delta variants relative to Alpha variant in individuals with infection-induced immunity”.
https://pubmed.ncbi.nlm.nih.gov/35862508/
215) Borsche et al.: COVID-19 Mortality Risk Correlates Inversely with Vitamin D3 Status, and a Mortality Rate Close to Zero Could Theoretically Be Achieved at 50 ng/mL 25(OH)D3: Results of a Systematic Review and Meta-Analysis. Nutrients, 2021 Oct; 13(10): 3596.
”Conclusions: The datasets provide strong evidence that low D3 is a predictor rather than just a side effect of the infection. Despite ongoing vaccinations, we recommend raising serum 25(OH)D levels to above 50 ng/mL to prevent or mitigate new outbreaks due to escape mutations or decreasing antibody activity”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8541492/
216) Cardozo et al.: Informed consent disclosure to vaccine trial subjects of risk of COVID-19 vaccines worsening clinical disease. International Journal of Clinical Practice, 2021 Mar;75(3):e13795.
“COVID-19 vaccines designed to elicit neutralising antibodies may sensitise vaccine recipients to more severe disease than if they were not vaccinated… these vaccines… may worsen COVID-19 disease via antibody-dependent enhancement (ADE)… The specific and significant COVID-19 risk of ADE should have been and should be prominently and independently disclosed to research subjects currently in vaccine trials, as well as those being recruited for the trials and future patients after vaccine approval, in order to meet the medical ethics standard of patient comprehension for informed consent”.
https://pubmed.ncbi.nlm.nih.gov/33113270/
217) Shelley et al: Impact of Microbiota: A Paradigm for Evolving Herd Immunity against Viral Diseases. Viruses, 2020 Oct; 12(10): 1150.
“Here, we outline several factors that can directly or indirectly alter the acquisition of immunity. Among those factors, the gut microbiota is an essential element that contributes to shaping individual immunity and thereby mapping the population immunity. Gut microbiota, along with nutrients and environmental factors, play an indispensable and cumulative role in establishing herd immunity against any infectious disease. We propose that heterologous immunity is the most important factor contributing protection against SARS-CoV-2 infection in India… Imbalance of nutrients perturbs microbiota and abrogates immunity. Thus, a particular population can become vulnerable to the infection… Intestinal dysbiosis leads to environmental enteropathy (EE). As a consequence, the generation of herd immunity can either be delayed or not start in a particular cohort”. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7599628/
218) Sanders: Stronger, More Robust Natural Immunity Thwarts Any Case for “Vaccine Passports”. American Institute for Economic Research, September 9, 2021.
“A growing body of research is making it increasingly clear that natural immunity to Covid-19 owing to previous infection is stronger, more durable, and broader than vaccine-induced immunity. Apart from not being unusual among infectious diseases, this fact has significant implications for governmental, school, employer, and business plans to harass and restrict people who aren’t vaccinated”.
https://www.aier.org/article/stronger-more-robust-natural-immunity-thwarts-any-case-for-vaccine-passports/?gclid=EAIaIQobChMI-uChscjc9QIV8EaRBR234AeWEAMYAyAAEgJuEPD_BwE
219) Sekine et al: Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell, 2020 Oct 1;183(1):158-168.
“Our collective dataset shows that SARS-CoV-2 elicits broadly directed and functionally replete memory T cell responses, suggesting that natural exposure or infection may prevent recurrent episodes of severe COVID-19”.
https://pubmed.ncbi.nlm.nih.gov/32979941/
220) Corona passport changes: Previous infection protects worse than vaccine. Berlingske, 11.11.2021
https://www.berlingske.dk/danmark/coronapas-aendres-tidligere-smitte-beskytter-daarligere-end-vaccine (text in Danish)
The Danish Health Authority recommends a shorter duration of the corona passport
https://www.sst.dk/da/nyheder/2022/sundhedsstyrelsen-anbefaler-kortere-varighed-af-coronapasset (text in Danish)
221) Trougakos et al: Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends in Molecular Medicine, 2022 Jul; 28(7): 542–554.
“Adverse effects (AEs) following vaccination have been noted which may relate to a proinflammatory action of the lipid nanoparticles used or the delivered mRNA (i.e., the vaccine formulation), as well as to the unique nature, expression pattern, binding profile, and proinflammatory effects of the produced antigens – spike (S) protein and/or its subunits/peptide fragments – in human tissues or organs…
In mRNA vaccines, which are characterized by relatively rapid prototyping and manufacturing on a large scale, the S protein-encoding mRNA is delivered via lipid nanoparticles (LNPs) to human cells that produce the mature viral protein or related antigens…
Finally, as we essentially do not know for how long and at what concentration the LNPs and the antigen(s) remain in human tissues or the circulation of poor vaccine responders, the elderly, or children (see Outstanding questions), and given the fact that cellular immunity likely persists despite reduced in vitro neutralizing titers [28], boosting doses should be delivered only where the benefit–risk profile is clearly established”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9021367/
222) Ntouros et al: Effective DNA damage response after acute but not chronic immune challenge: SARS-CoV-2 vaccine versus Systemic Lupus Erythematosus. Clinical Immunology, 2021 Aug; 229: 108765.
“Whether and how an acute immune challenge may affect DNA Damage Response (DDR) is unknown. By studying vaccinations against Influenza and SARS-CoV-2 (mRNA-based) we found acute increases of type-I interferon-inducible gene expression, oxidative stress and DNA damage accumulation in blood mononuclear cells of 9 healthy controls, coupled with effective anti-SARS-CoV-2 neutralizing antibody production in all.
Increased DNA damage after SARS-CoV-2 vaccine, partly due to increased oxidative stress, was transient, whereas the inherent DNA repair capacity was found intact.
In contrast, in 26 patients with Systemic Lupus Erythematosus, who served as controls in the context of chronic immune activation, we validated increased DNA damage accumulation, increased type-I interferon-inducible gene expression and induction of oxidative stress, however aberrant DDR was associated with deficiencies in nucleotide excision repair pathways. These results indicate that acute immune challenge can indeed activate DDR pathways, whereas, contrary to chronic immune challenge, successful repair of DNA lesions occurs”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8171000/
223) Kowarz et al: Vaccine-induced COVID-19 mimicry syndrome. Elife, Cell Biology, Microbiology and infectious Disease, Jan 27, 2022.
”Meanwhile, scientists have proposed an immune-based pathomechanism and the condition has been coined vaccine-induced immune thrombotic thrombocytopenia (VITT)”.
https://elifesciences.org/articles/74974
224) Harvard researchers: COVID-19 vaccines could permanently alter human genomic DNA
https://www.imuno-medica.ro/news/Harvard-researchers-say-COVID-19-vaccines-could-permanently-alter-human-genomic-DNA-29
225) Danmark har 8,8% ikke-vestlige indvandrere (2020) (Denmark has 8.8% non-Western immigrants (2020))
https://integrationsbarometer.dk/tal-og-analyser/INTEGRATION-STATUS-OG-UDVIKLING
226) Sverige har 22% indvandrere (2020) (Sweden has 22% immigrants (2020)
https://www.altinget.dk/sundhed/artikel/kronik-rammer-coronavirussen-isaer-indvandrere-og-fattige
https://jyllands-posten.dk/debat/blogs/mortenjensen/ECE10023884/206-procent-i-sverige/
227) Kanstrup og Petersen, København Universitet: Danmark og Sverige har »klaret sig fuldstændig ens – trods vidt forskellige strategier. (Denmark and Sweden have ‘done exactly the same – despite very different strategies)
Berlingske, den 08.7.2022.
https://www.berlingske.dk/kronikker/professor-og-virolog-om-doedstal-under-corona-danmark-og-sverige-har
228) Sundhedsmagasinet: Danmark kan spare 15-16% (30-32 mia. kr.) på de årlige sundhedsudgifter, ved at udrydde D-vitaminmangel, Dr tv, 2016.
(Sundhedsmagasinet: Denmark can save 15-16% (DKK 30-32 billion) on annual health costs by eradicating vitamin D deficiency, Dr tv, 2016).
229) 15% more elderly people over 80 in Sweden compared to Denmark. (15% flere ældre over 80 år i Sverige ift. Danmark). Nordic Counsil of Minister: State of the Nordic Region, 201815, figur 2.4, side 28.
http://norden.diva-portal.org/smash/get/diva2:1180241/FULLTEXT01.pdf
230) EBMPHET Consortium: COVID-19 Severity in Europe and the USA: Could the Seasonal Influenza Vaccination Play a Role? EBMPHET Consortium, June 7, 2020 (pre-print)
”Our analysis indicates that receiving seasonal influenza vaccination(s) in the past might be an additional risk factor for the elderly in terms of enhanced susceptibility to infection with SARS-CoV-2 and higher likelihood of a lethal outcome in case of infection. More research about this possible risk factor is urgently needed”.
https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3621446
231) Kim et al: Duration of SARS-CoV-2 Natural Immunity and Protection against the Delta Variant: A Retrospective Cohort Study. Clinical Infectious Diseases, 2021 Dec 3 : ciab999.
”SARS-CoV-2 infection is highly protective against reinfection with Delta. Immunity from prior infection lasts at least 13 months. Countries facing vaccine shortages should consider delaying vaccinations for previously infected patients to increase access”.
https://pubmed.ncbi.nlm.nih.gov/34864907/
232) Flacco et al: Risk of reinfection and disease after SARS-CoV-2 primary infection: Meta-analysis. European Journal of Clinical investigation, 2022 Oct;52(10):e13845
”A strong natural immunity follows the primary infection and may last for more than one year, suggesting that the risk and health care needs of recovered subjects might be limited. Although the reinfection rates considerably increased during the Omicron wave, the risk of a secondary severe or lethal disease remained very low. The risk-benefit profile of multiple vaccine doses for this subset of population needs to be carefully evaluated”.
https://pubmed.ncbi.nlm.nih.gov/35904405/
(Meta-analysis, involving 15 million people)
233) Gazit et al: Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Naturally Acquired Immunity versus Vaccine-induced Immunity, Reinfections versus Breakthrough Infections: A Retrospective Cohort Study. Clinical Infectious Diseases, 2022 Aug 24;75(1):e545-e551.
”Methods: A retrospective observational study of 124 500 persons, compared 2 groups: (1) SARS-CoV-2-naive individuals who received a 2-dose regimen of the BioNTech/Pfizer mRNA BNT162b2 vaccine, and (2) previously infected individuals who have not been vaccinated.
Results: SARS-CoV-2-naive vaccinees had a 13.06-fold (95% confidence interval [CI], 8.08-21.11) increased risk for breakthrough infection with the Delta variant compared to unvaccinated-previously-infected individuals, when the first event (infection or vaccination) occurred during January and February of 2021. The increased risk was significant for symptomatic disease as well. When allowing the infection to occur at any time between March 2020 and February 2021, evidence of waning naturally acquired immunity was demonstrated, although SARS-CoV-2 naive vaccinees still had a 5.96-fold (95% CI: 4.85-7.33) increased risk for breakthrough infection and a 7.13-fold (95% CI: 5.51-9.21) increased risk for symptomatic disease.
Conclusions: Naturally acquired immunity confers stronger protection against infection and symptomatic disease caused by the Delta variant of SARS-CoV-2, compared to the BNT162b2 2-dose vaccine-indued immunity”.
https://pubmed.ncbi.nlm.nih.gov/35380632/
234) Ridgway et al: Rates of COVID-19 Among Unvaccinated Adults With Prior COVID-19. Jama Network, JAMA Netw Open. 2022;5(4):e227650
“Among 121 615 patients with more than 10 million days of follow-up, unvaccinated individuals with prior symptomatic COVID-19 had 85% lower risk of acquiring COVID-19 than unvaccinated individuals without prior COVID-19. Prior studies investigating protection against SARS-CoV-2 reinfection found similar results, with protection associated with natural immunity ranging from 80.5% to 100%”.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2791312
235) Verschoor et al: Natural immunity helps overcome the age-related decline of SARS-CoV-2 vaccine immunogenicity. The Lancet, July 2022
“Regarding SARS-CoV-2, most studies support this idea, with evidence of higher antibody responses in previously infected compared to uninfected vaccinees. In line with this, vaccine effectiveness against infection following second dose in previously uninfected adults declines from 85% after 1–2 months to 51% approximately 9 months later, whereas for those previously infected, effectiveness remains around 90% after 9 months”.
https://www.thelancet.com/journals/lanhl/article/PIIS2666-7568(22)00146-5/fulltext
236) Pugh et al: The unnaturalistic fallacy: COVID-19 vaccine mandates should not discriminate against natural immunity. British Medical Journal, Journal of Medical Ethics, Volume 48, Issue 6
”At the present time, however, it appears that requiring those with natural immunity to undergo vaccination is not clearly necessary to obtain a substantial public health benefit. If so, it is discriminatory to treat natural immunity differently to vaccine-mediated immunity, and this is something that ethical, evidence-based public health policy should reflect”.
https://jme.bmj.com/content/48/6/371
237)Townsend et al: The durability of natural infection and vaccine-induced immunity against future infection by SARS-CoV-2. PNAS, 2022 Aug 2; 119(31):
”Vaccines typically go through several phases of clinical trials prior to deployment. However, during this time, viral antigens will continue to accrue new sequence variation that will gradually or abruptly shift them away from the target of the vaccine. Consequently, the immune response to a vaccine will, by necessity, target an earlier strain of the pathogen than would the immune response to a contemporaneous natural infection. This difference in strain targeting will mean that immune evasion by the virus will likely be more advanced against immunity conveyed by a vaccine than against immunity conveyed by natural infection. Indeed, neutralization efficacy of the ancestrally targeted SARS-CoV-2 vaccines against successive selected strains declined precipitously against the insurgent delta and omicron variants (57), and natural infection by a predominant strain has been shown to update vaccine-mediated immune targeting, increasing the antigenic breadth of neutralizing capacity”.
https://www.pnas.org/doi/full/10.1073/pnas.2204336119
238) Margiotti et al: Natural immune response and protection from SARS-CoV-2 reinfection. Acta Virologica Vol.65, No.4, p.333-338, 2021
”Although more studies are needed, evidence suggests that virus-specific B cell response in people with SARS-CoV-2 infection is rapidly generated and seems to be more reliable marker of long-lasting humoral responses than serum antibodies”.
https://pubmed.ncbi.nlm.nih.gov/34796710/
239) Chemaitelly et al: Duration of immune protection of SARS-CoV-2 natural infection against reinfection. Journal of Travel Medicine, 2022 Sep 30;taac109
”Protection of natural infection against reinfection wanes and may diminish within a few years. Viral immune evasion accelerates this waning. Protection against severe reinfection remains very strong, with no evidence for waning, irrespective of variant, for over 14 months after primary infection”.
https://pubmed.ncbi.nlm.nih.gov/36179099/
240) Mischlmayr et al: Observed protection against SARS-CoV-2 reinfection following a primary infection: A Danish cohort study among unvaccinated using two years of nationwide PCR-test data. The Lancet Regional Health – Europe, 2022 Sep; 20: 100452
”SARS-CoV-2 infection offered a high level of sustained protection against reinfection, comparable with that offered by vaccines…
The level of estimated protection against serious disease was somewhat higher than that against infection and possibly longer lasting”.
https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(22)00146-6/fulltext
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9245510/
241) Flacco et al: Risk of SARS-CoV-2 Reinfection 18 Months After Primary Infection: Population-Level Observational Study. Frontiers in Public Health, 2022 May 2;10:884121
“…the incidence of reinfection did not vary substantially over time: after 18-22 months from the primary infection, the reinfection rate was still 6.7‰, suggesting that protection conferred by natural immunity may last beyond 12 months. The risk of reinfection was significantly higher among females, unvaccinated subjects, and during the Omicron wave”.
https://pubmed.ncbi.nlm.nih.gov/35586006/
242) Carazo et al: Estimated Protection of Prior SARS-CoV-2 Infection Against Reinfection With the Omicron Variant Among Messenger RNA–Vaccinated and Nonvaccinated Individuals in Quebec, Canada. Jama Network open, 2022 Oct; 5(10): e2236670.
“The findings of this study suggest that vaccination with 2 or 3 mRNA vaccine doses among individuals with prior heterologous SARS-CoV-2 infection provided the greatest protection against Omicron-associated hospitalization. In the context of program goals to prevent severe outcomes and preserve health care system capacity, a third mRNA vaccine dose may add limited protection in twice-vaccinated individuals with prior SARS-CoV-2 infection”.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2797311
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9568797/
243) Diani et al: SARS-CoV-2-The Role of Natural Immunity: A Narrative Review. Journal of Clinical Medicine, 2022 Oct 25;11(21):6272.
“This extensive narrative review regarding a vast number of articles highlighted the valuable protection induced by the natural immunity after COVID-19, which seems comparable or superior to the one induced by anti-SARS-CoV-2 vaccination”.
https://pubmed.ncbi.nlm.nih.gov/36362500/
244) Chowdhury et al: Immune response in COVID-19: A review. Journal of infection and Public Health, 2020 Nov; 13(11): 1619–1629.
“According to the World Health Organization, healthy foods and hydration are vital. Individuals consuming a well-balanced diet are healthier with a strong immune system and have a reduced risk of chronic illness, infectious diseases. Vitamins and minerals are vital”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7359800/
245) Abu-Saleh et al: Neutralizing antibodies against SARS-CoV-2 are higher but decline faster in mRNA vaccinees compared to individuals with natural infection. Journal of Travel Medicine, 2022 Nov 7;taac130
“While double-dose mRNA vaccination boosted antibody levels, vaccinated individuals’ ‘boost’ was relatively short-lived”.
https://pubmed.ncbi.nlm.nih.gov/36342115/
246) Chemaitelly: Efficacy of Natural Immunity against SARS-CoV-2 Reinfection with the Beta Variant. New England Journal of Medicine, 2021 Dec 15 : NEJMc2110300.
“Protection by previous SARS-CoV-2 infection against reinfection with the beta variant was observed, even 1 year after the primary infection, but protection was slightly lower than that against the alpha variant and wild-type virus circulating in Qatar.3-5 These findings give some insights into the hypothesis that natural immunity may provide protection against known variants of concern”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8693689/
247) Yan et al: Neutralizing Antibodies and Cellular Immune Responses Against SARS-CoV-2 Sustained One and a Half Years After Natural Infection. Frontiers in Microbiology, 2022 Mar 3;12:803031.
”We found that 91.9% (57/62) and 88.9% (40/45) of COVID-19 patients had NAbs against SARS-CoV-2 in a year (10-11 months) and one and a half years (17-18 months)… We concluded that SARS-CoV-2 infection elicited a robust and persistent neutralizing antibody and memory T-cell response in COVID-19 patients, indicating that these sustained immune responses, among most SARS-CoV-2-infected people, may play a crucial role in protection against reinfection”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8928406/
248) Peres-Aslos et al: Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors, Nature Communications, 2022; 13: 1614.
“Our results indicate that IgG levels will drop at different rates depending on prior infection, age, sex, T-cell response, and the interval between vaccine injections. Only natural infection mounted a significant and lasting IgA response”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8960902/
249) Addo et al: Duration of immunity following full vaccination against SARS-CoV-2: a systematic review. Archives of Public Health, 2022; 80: 200.
“Waning of immunity against SARS-CoV-2 begins as early as the first month after full vaccination and this decline continues till the sixth month when the level of immunity may not be able to provide adequate protection against SARS-CoV-2”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9436729/
250) Larenas-Linnemann et al: Enhancing innate immunity against virus in times of COVID-19: Trying to untangle facts from fictions. World Alliance Organization Journal, 2020 Nov; 13(11): 100476.
”Without the intention to write an official systematic review, but more to give an overview of possible strategies, in this review article we discuss several interventions that might stimulate innate immunity and thus our defense against (viral) respiratory tract infections. Some of these interventions can also stimulate the adaptive T- and B-cell responses, but our main focus is on the innate part of immunity. We divide the reviewed interventions into: 1) lifestyle related (exercise, >7 h sleep, forest walking, meditation/mindfulness, vitamin supplementation); 2) Non-specific immune stimulants (letting fever advance, bacterial vaccines, probiotics, dialyzable leukocyte extract, pidotimod), and 3) specific vaccines with heterologous effect (BCG vaccine, mumps-measles-rubeola vaccine, etc).
Results
For each of these interventions we briefly comment on their definition, possible mechanisms and evidence of clinical efficacy or lack of it, especially focusing on respiratory tract infections, viral infections, and eventually a reduced mortality in severe respiratory infections in the intensive care unit. At the end, a summary table demonstrates the best trials supporting (or not) clinical evidence.
Conclusion
Several interventions have some degree of evidence for enhancing the innate immune response and thus conveying possible benefit, but specific trials in COVID-19 should be conducted to support solid recommendations”.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7546230/
251) Gibbons et al: Association between vitamin D supplementation and COVID-19 infection and mortality. Scientific Reports, 2022 Nov 12;12(1):19397.
“Vitamin D deficiency has long been associated with reduced immune function that can lead to viral infection. Several studies have shown that Vitamin D deficiency is associated with increases the risk of infection with COVID-19. However, it is unknown if treatment with Vitamin D can reduce the associated risk of COVID-19 infection, which is the focus of this study. In the population of US veterans, we show that Vitamin D2 and D3 fills were associated with reductions in COVID-19 infection of 28% and 20%, respectively [(D3 Hazard Ratio (HR) = 0.80, [95% CI 0.77, 0.83]), D2 HR = 0.72, [95% CI 0.65, 0.79]]. Mortality within 30-days of COVID-19 infection was similarly 33% lower with Vitamin D3 and 25% lower with D2 (D3 HR = 0.67, [95% CI 0.59, 0.75]; D2 HR = 0.75, [95% CI 0.55, 1.04]). We also find that after controlling for vitamin D blood levels, veterans receiving higher dosages of Vitamin D obtained greater benefits from supplementation than veterans receiving lower dosages. Veterans with Vitamin D blood levels between 0 and 19 ng/ml exhibited the largest decrease in COVID-19 infection following supplementation. Black veterans received greater associated COVID-19 risk reductions with supplementation than White veterans. As a safe, widely available, and affordable treatment, Vitamin D may help to reduce the severity of the COVID-19 pandemic”.
https://pubmed.ncbi.nlm.nih.gov/36371591/

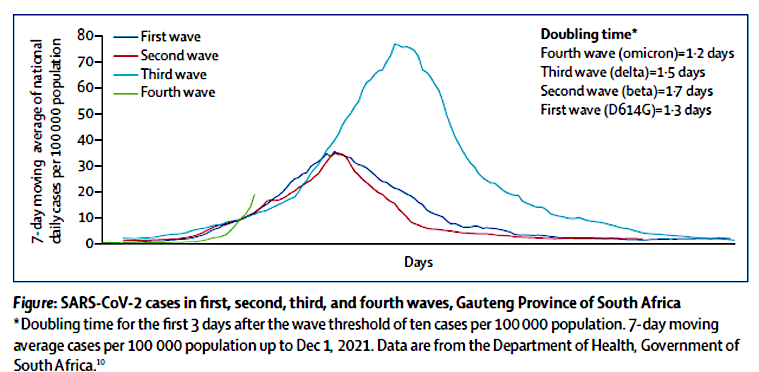
 Figure 6. Number of Omicron cases in DK weeks 47-49.5 according to SSI.
Figure 6. Number of Omicron cases in DK weeks 47-49.5 according to SSI.
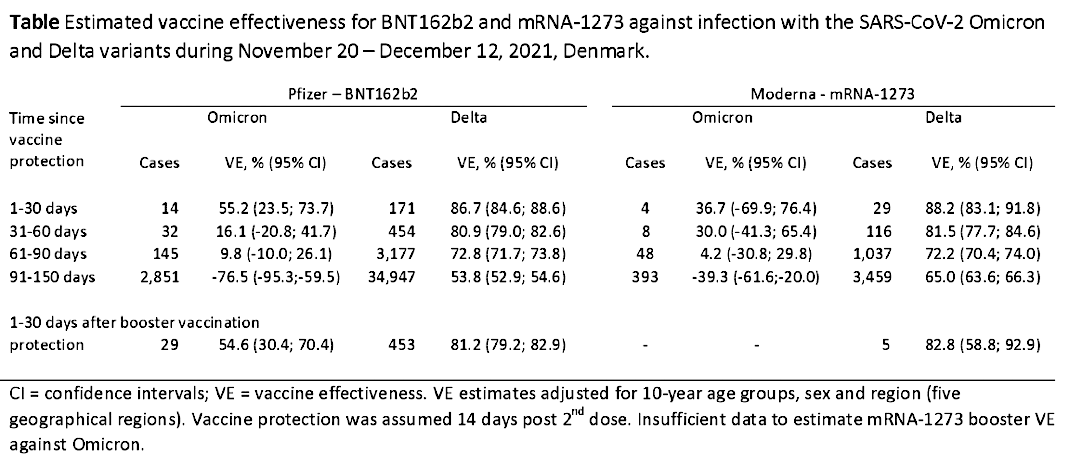
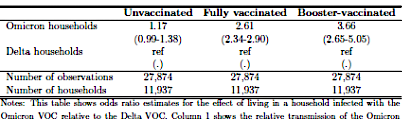
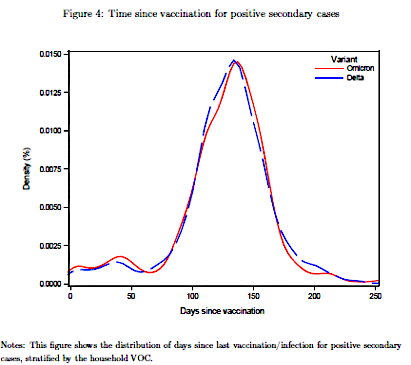
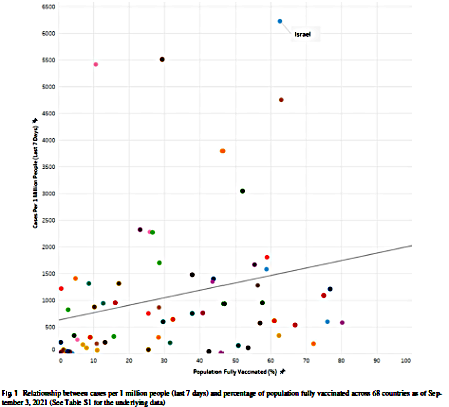

 The other day, I heard the happy voice of a radio host on DR4 tell me that the Omicron infection is rising and rising, while the Delta variant has slowly begun to decline.
The other day, I heard the happy voice of a radio host on DR4 tell me that the Omicron infection is rising and rising, while the Delta variant has slowly begun to decline.